Abstract
The development of refractory tumor cells limits therapeutic efficacy in cancer by activating mechanisms that promote cellular proliferation, migration, invasion, metastasis, and survival. Benzimidazole anthelmintics have broad-spectrum action to remove parasites both in human and veterinary medicine. In addition to being antiparasitic agents, benzimidazole anthelmintics are known to exert anticancer activities, such as the disruption of microtubule polymerization, the induction of apoptosis, cell cycle (G2/M) arrest, anti-angiogenesis, and blockage of glucose transport. These antitumorigenic effects even extend to cancer cells resistant to approved therapies and when in combination with conventional therapeutics, enhance anticancer efficacy and hold promise as adjuvants. Above all, these anthelmintics may offer a broad, safe spectrum to treat cancer, as demonstrated by their long history of use as antiparasitic agents. The present review summarizes central literature regarding the anticancer effects of benzimidazole anthelmintics, including albendazole, parbendazole, fenbendazole, mebendazole, oxibendazole, oxfendazole, ricobendazole, and flubendazole in cancer cell lines, animal tumor models, and clinical trials. This review provides valuable information on how to improve the quality of life in patients with cancers by increasing the treatment options and decreasing side effects from conventional therapy.
Keywords: Benzimidazole, Anthelmintics, Cancer, Therapeutics
INTRODUCTION
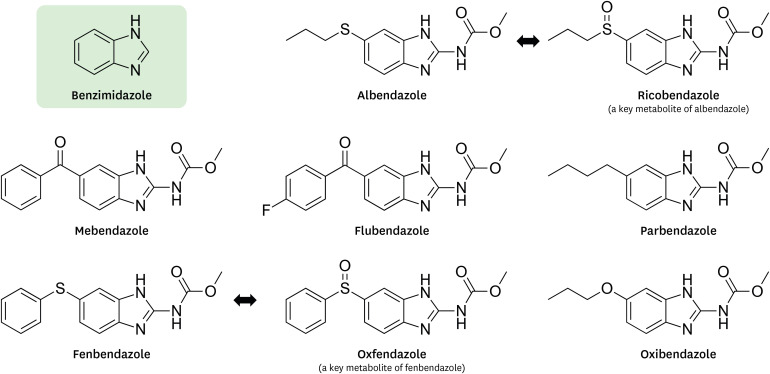
Benzimidazole anthelmintics have a chemical structure of benzimidazole with broad anthelmintic activity and are extensively used in both human and veterinary medicine to control internal parasites. Due to their low cost and high efficacy, benzimidazole anthelmintics have been used throughout the world since their introduction in the 1960s (1). Benzimidazole anthelmintics are well-tolerated without severe side effects, and their decades of use provide a basis for safety in humans. Benzimidazole anthelmintics selectively bind to β-tubulin of parasitic worms, causing their immobilization and death. The binding of benzimidazole anthelmintics to nematode tubulin had a 250–400-fold greater inhibition compared with that of mammals (2), indicating selective toxicity toward parasites. Due to the selective interaction with microtubules, benzimidazole anthelmintic drugs have been extensively studied as antitumor agents to disrupt tubulin polymerization for drug repurposing. In this review, we focus on the antitumor effects of the following benzimidazole anthelmintics for human or veterinary use: albendazole, parbendazole, oxibendazole, ricobendazole, mebendazole, fenbendazole, oxfendazole, and flubendazole (Fig. 1).
Figure 1. Structures of benzimidazole anthelmintics.
Conventional cancer therapy, including chemotherapy and radiation therapy, appears to have severe toxic effects, such as normal cell death, neurotoxicity, cardiotoxicity, gastrointestinal toxicity, and immune suppression, resulting in a diminished quality of life in patients with cancer (3). Furthermore, chemotherapeutic drugs most often are associated with resistance and eventual evasion of their cytotoxic effects. The benzimidazole anthelmintics are known to be effective in inhibiting paclitaxel and doxorubicin-resistant cancer cells (4), overcoming multidrug resistance. Based on antitumor activity in cancer cells, benzimidazole anthelmintics may provide adjuvant and neoadjuvant therapy in combination with conventional therapy, leading to increased treatment success and decreased risk of recurrence. As benzimidazole anthelmintics have a strong safety profile, combination therapy may decrease the toxic effects of conventional therapy by adjusting the dosage of chemotherapeutics with high doses of these anthelmintics. However, all benzimidazoles have low aqueous solubility and poor absorption from the gastrointestinal tract; the low bioavailability of benzimidazole anthelmintics may significantly improve their absorption by coadministration with a fatty meal (5). Strategies to overcome the low bioavailability of benzimidazole anthelmintics help to increase the antitumor effects by adopting various formulations, including the use of liposomes, nanoparticles, and cyclodextrins. This review article covers the antitumor activity of benzimidazole anthelmintics in cell lines, animal tumor models, and clinical trials. Finally, the pharmacokinetic properties and side effects of benzimidazole anthelmintics are briefly summarized based on reviews and websites, providing a basic understanding to improve the use of benzimidazole anthelmintics in the cancer field. Furthermore, additional benzimidazole derivatives that have been developed for other purposes, such as anticancer, antimicrobial, and antidiabetic agents, are excluded in this review, as they are out of scope.
ANTITUMORIGENIC EFFECTS OF BENZIMIDAZOLE ANTHELMINTICS IN CANCER CELLS
Supplementary Table 1 (4,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80) summarizes the antitumorigenicity of benzimidazole anthelmintics on cancer cell lines. Benzimidazole anthelmintics inhibit the progression of cancer through a variety of mechanisms. The most common antitumor effects of benzimidazole anthelmintics are: inhibited cell viability, migration, and invasion; reduced colony formation, disrupted tubulin polymerization, induced apoptosis and autophagy, increased sub G1, G2/M cell cycle arrest, induced differentiation and senescence, multinucleation, reduced angiogenesis, inhibited drug resistance and transporters, and impaired glucose utilization. Benzimidazole anthelmintics have been shown to inhibit cell viability in a variety of cancer cell lines, appearing as a promising medication (14). The half maximal inhibitory concentrations (IC50s) of flubendazole were well described in 461 cancer cell lines. Cell growth inhibition above 5 µM of fenbendazole are as follows: hepatocellular carcinoma (HCC) 70, HCC1143, HCC1937, and MCF-7 cells in breast cancer; LN405, TU132, U138MG, and U87MG cells in glioma; HUH6 clone5, HUH7, and SK-HEP-1 cells in hepatocellular carcinoma; P12-ICHIKAWA cells in leukemia; DMS79, H2228, and NCI-H146 cells in lung cancer; ONS76 cells in medulloblastoma; Rh28 cells in rhabdomyosarcoma; and T24 cells in urothelial carcinoma (14). These results indicate that some cancer cell lines may be less sensitive to benzimidazole anthelmintics. Even the same cell lines have differences in IC50s among benzimidazole anthelmintics, as well as laboratories which evaluated the IC50s (Supplementary Table 1). Identifying resistant and sensitive cancer cells is critical for the therapeutic application of benzimidazole anthelmintics in cancer types. Normal human lung fibroblasts and human umbilical vein endothelial cells have less sensitivity to mebendazole compared with lung cancer cells (56,57). Mebendazole had no effects on normal fibroblasts compared with adrenocortical carcinoma cells (79). The inhibitory effect of flubendazole was limited in normal human liver cells and cardiomyocytes compared with colorectal cancer cells (34). Fenbendazole showed little effect on normal human bronchial epithelial cells, normal immortalized human prostate cells, and primary mouse fibroblasts compared with lung cancer cells, as the IC50 values for fenbendazole were several fold higher in these normal cells compared with cancer cell lines (38). These results indicate that benzimidazole anthelmintics are relatively non-toxic to normal cells, increasing specific sensitivity to cancer cells. The benzimidazole anthelmintics usually decreased signaling proteins related to cell proliferation and survival, such as phosphorylated (p)HER2/3, PI3K/protein kinase B (AKT), rapidly accelerated fibrosarcoma (a family of three serine/threonine-specific protein kinases) (RAF)/MEK/ERK, mTOR, and Ki-67. Interestingly, fenbendazole inhibited PI3K/AKT and RAF/MEK/ERK in Kirsten rat sarcoma viral oncogene homolog (KRAS)-mutant lung cancer cells but had no effects in KRAS-wild-type H-1650 lung cancer cells despite inhibited cell viability (53). Fenbendazole also had no effects on cell viability in some p53 mutant lung cancer cells (55). These findings indicate that mutation conditions in cancer cells may affect the sensitivity of benzimidazole anthelmintics differentially.
Among MAPK signaling, benzimidazole anthelmintics inhibited ERK activation (14,51) but increased p38 and c-Jun N-terminal kinases (JNK) activations (38,55). As benzimidazole anthelmintics increased ELK1/serum response factor (SRF) to activate the Fos family, AP-1 also was activated, likely due to the activation of the Jun family from increased pJNK with the activated Fos family (45).
Benzimidazole anthelmintics inhibited cell migration and invasion, which are related to an epithelial-to-mesenchymal transition (EMT). The benzimidazole anthelmintics decreased N-cadherin, vimentin, FAK, and matrix metalloproteinase (MMP) 2, leading to inhibition of the mesenchymal phase followed by reduced metastasis. The benzimidazole anthelmintics decreased β-tubulin in cancer cells. Although having a 250–400-fold greater inhibition in the tubulin of parasites compared with that of mammals (2), benzimidazole anthelmintics disrupted tubulin polymerization broadly in cancer cells, leading to a lethal effect in rapidly dividing cells. The disruption of tubulin polymerization brings multinucleation and mitotic catastrophe in cancer cells. Interestingly, albendazole had no effects on tubulin depolymerization in HCT116 colorectal cancer cells despite its inhibited cell viability (36,37).
Benzimidazole anthelmintics induced autophagy as they increased autophagic proteins, such as microtubule-associated protein 1 light chain 3 (LC3) and Beclin-1. Mebendazole induces autophagy in endothelial cells (81), likely impairing angiogenic activity via decrease in Wnt/β-catenin axis and VEGFR-2 levels (82). Autophagy has a dual role in tumorigenesis regulation (83). Although autophagy facilitates cell survival in tumor initiation, it suppresses tumor promotion through mTOR and ROS downregulation after tumor formation (83,84). Autophagy promotes invasion of cancer stem cells through TGF-1β dependent epithelial-mesenchymal transition (85). However, autophagy inhibitors have not yet shown survival improvements in clinical trials (86). Further studies require to clarify mechanisms by which benzimidazole anthelmintics induce autophagy.
Above all, benzimidazole anthelmintics induced apoptosis by increasing sub G1 phase, caspase-3/7/8/9 activity and cleaved poly(ADP-ribose) polymerase (PARP), B-cell lymphoma 2 (Bcl-2)-associated X protein (Bax; pro-apoptotic), cytochrome c, p53 upregulated modulator of apoptosis (PUMA), and γH2AX, while decreasing Bcl-2 (anti-apoptotic), truncated Bid (pro-apoptotic), pBAD (anti-apoptotic), myeloid cell factor-1 (Mcl-1), X-linked inhibitor of apoptosis protein (XIAP), and survivin (14,46).
The benzimidazole anthelmintics arrested G2/M phase specifically in the cell cycle by increasing phospho-Histone H3 (pH3), cyclin B1, and p27Kip1, while decreasing cyclin A, cyclin D1, cyclin E, CDC25c, and protein phosphatase 2A (PP2A) cα. Although parbendazole arrested G2/M phase, it decreased cyclin B1 in pancreatic cancer cells (68). The benzimidazole anthelmintics reduced angiogenesis-related proteins, such as hypoxia-induced hypoxia-inducible factor (HIF)-1α and VEGF (54). The benzimidazole anthelmintics induced differentiation and senescence, attributing to increased Keratin 18 and p53 tumor suppressor. The p53 wild-type cancer cells have a higher sensitivity to benzimidazole anthelmintics compared with the p53 mutant cells (38). Benzimidazole anthelmintics increased pp53, p53, and p21 but decreased mutant p53, mouse double minute 2 homolog (Mdm2; sometimes increased), mouse double minute 4 (MdmX), CDK4, CDK6, and pRB (22,23,58,59).
The benzimidazole anthelmintics induced cellular stress through the accumulation of ubiquitylated proteins and increased ROS levels, C/EBP homologous protein (CHOP), activating transcription factor (ATF) 4, and caspase-4/12 as endoplasmic reticulum (ER) stress markers (72).
They also reduced colony formation and inhibited stemness in cancer cells, attributing to decreased a human homology with the viral gene v-myc (c-MYC), OCT4, SRY (sex determining region Y)-box 2 (SOX2), NANOG, aldehyde dehydrogenase 1 (ALDH1) activity, the CD24low/CD44high and CD24high/CD49high subpopulation as stem-like cells, and CD44 as stem cell markers (20).
Benzimidazole anthelmintics impaired glucose utilization, decreasing glucose transporter (GLUT)-1, ATP production, hypoxia-induced glycolysis, GLUT-4, and hexokinase II. These decreases indicate that benzimidazole anthelmintics may inhibit the Warburg effect in cancer. Furthermore, benzimidazole anthelmintics increased AMP-activated protein kinase (AMPK) activation (15), which negatively regulate the Warburg effect. However, fenbendazole had no change in glucose uptake in OCI-AML2 leukemia cells (30), indicating that effects of benzimidazole anthelmintics on glucose metabolisom are a cell-type-specific manner. The benzimidazole anthelmintics inhibited genes related to drug resistance and transporters, such as MDR1, ABCB1, ABCC1, SLC47A1, and p62 (41,42,43).
The benzimidazole anthelmintics inhibited NF-κB signaling by decreasing phospho-p65 and the nuclear translocation of NF-κB and by increasing the half-life of nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (IκBα). However, albendazole-induced sirtuin 3 (SIRT3) degradation elicited ROS-mediated p38 MAPK activation, leading to pyruvate kinase M2-mediated tristetraprolin (TTP) degradation, followed by TNF-α upregulation in human leukemia cells (46). The benzimidazole anthelmintics inhibited hedgehog signaling, likely leading to reduced metastasis, inhibited angiogenesis, and increased apoptosis. The benzimidazole anthelmintics inhibited pSTAT3 and nuclear translocation of STAT3, resulting in decreased proliferation, increased apoptosis, reduced angiogenesis, downregulated stemness, increased chemosensitivity, and immune surveillance (34,38). On the other hand, they increased STAT1/2, inducing cell death. The benzimidazole anthelmintics inhibited c-Src activation, resulting in decreased tumor growth and metastasis. Flubendazole decreased PD-1 in melanoma cells (76), likely inhibiting tumor growth.
The benzimidazole anthelmintics decreased β-catenin, maintaining the Wnt-off state. Mebendazole is a novel TRAF2- and NCK-interacting kinase (TNIK) inhibitor (87). As TNIK is an activating kinase for the T-cell factor-4 (TCF4) and β-catenin transcriptional complex (88), benzimidazole anthelmintics may impede the Wnt signaling by inhibiting TNIK. The benzimidazole anthelmintics increased AMPK activation, likely resulting in inhibited mTOR, decreased cyclin D1, and increased p53 and p21. Oxibendazole increased microRNA (miR)-24a and miR-204 in prostate cancer cells (71). Although miR-24a repressed apoptosis in the developing neural retina (89), miR-204 inhibits invasion and EMT in esophageal cancer (90). The effects of benzimidazole anthelmintics on miRNA signature in cancer are still immature. Fenbendazole is a more effective cytotoxic agent as compared with cisplatin and taxol (at IC50 concentration) and had similar effects also with the Food and Drug Administration (FDA)-approved proteasomal inhibitor bortezomib (38). The benzimidazole anthelmintics decreased proteasome activity, acting as a proteasomal inhibitor.
Mebendazole had little or no effect on clustering and cytokine release in non-activated PBMCs, but increased clustering and released proinflammatory cytokines, including TNF-α, IL-1β, IFN-γ, and IL-6 in CD3/IL2-activated PBMCs, potentiating both tumor cell reduction and apoptosis (91). Mebendazole induced a proinflammatory (M1) phenotype of monocytic cells (THP-1) via ERK1/2 and TLR8-dependent inflammasome activation (48). The differentiated THP-1 macrophages enhanced the suppressive effects of mebendazole in leukemia cells. These results may be relevant to explain the anticancer activity of benzimidazole anthelmintics.
Benzimidazole anthelmintics sensitize cancer cells to conventional therapy, such as chemotherapeutics and radiation, enhanceing antitumor potentials when used in combination. Benzimidazole anthelmintics show synergistic antitumorigenicity in combination with temozolomide, vinblastine, fluorouracil, doxorubicin, docetaxel, gemcitabine, paclitaxel, cisplatin, and trametinib.
Epothilone-paclitaxel resistant leukemic cells are sensitive to albendazole (49). These facts indicate that benzimidazole anthelmintics may be adjuvant therapeutics in cancer cells resistant to conventional chemotherapy. However, fenbendazole had no change in cytotoxicity in combination with docetaxel in EMT6 mouse mammary tumor cells (29). Albendazole showed antagonistic effects in combination with paclitaxel and vinblastine in HCT-116 colorectal cancer cells and DU145 prostate cancer cells (36). Mebendazole had antagonistic and additive cytotoxicity in combination with trametinib in STU (BRAFV600K/NRASWT) melanoma cells in a dose-dependent manner (75). These facts indicate the different sensitivity of benzimidazole anthelmintics in a cancer cell-type-specific manner, requiring the careful application of benzimidazole anthelmintics with conventional chemotherapy.
ANTITUMORIGENIC EFFECTS OF BENZIMIDAZOLE ANTHELMINTICS IN ANIMAL TUMOR MODELS
We summarized the antitumorigenicity of benzimidazole anthelmintics in animal tumor models (Supplementary Table 2) (4,8,11,12,13,14,15,16,17,20,27,28,29,30,34,35,36,37,39,45,47,51,52,54,55,56,57,61,63,64,65,66,69,70,71,74,75,76,78,79,80,92,93,94). Benzimidazole anthelmintics have been shown to inhibit the tumor growth in animals, which have been well-tolerated without significant side effects during the experimental period. Based on Supplementary Table 2, the most common antitumor effects of benzimidazole anthelmintics in animal tumor models appear as follows: prolonged overall survival and progression-free survival; inhibited tumor growth; reduced vessel formation; decreased tumor volume and weight; and reduced metastasis. The antitumor effects of benzimidazole anthelmintics in animal tumor models (Supplementary Table 2) share similar mechanisms with those in cancer cells (Supplementary Table 1). The benzimidazole anthelmintics in animal tumor models have shown the following mechanistic effects: decreased human epidermal growth factor receptor (HER) 2, pMEK, pERK1/2, pmTOR, fibroblast growth factor (FGF) 2, Ki-67, and proliferating cell nuclear antigen (PCNA) to inhibit tumor growth; increased LC3 and Beclin-1 to induce autophagy; increased caspase-3/9, Bax (pro-apoptotic), DNA fragmentation, apoptotic cells (transferase dUTP nick end labeling [TUNEL] staining) and p53+ cells, and decreased Bcl-2 (anti-apoptotic) and XIAP to trigger apoptosis; increased pH3 and decreased cyclin D1 to arrest cell cycle; decreased HIF-1α, VEGF, vascular endothelial growth factor receptor (VEGFR) 2 kinase activity, FGF2, and CD31 to reduce angiogenesis; reduced activities of hexokinase (HK), pyruvate kinase (PK), and LDH to impair glucose utilization; decreased vimentin, TGF-β, MMP2, MMP9, and MMP2/tissue inhibitor of metalloproteinase (TIMP) 1 ratio related to the inhibition of mesenchymal phase to reduce metastasis; decreased MYC, ALDH1A1, CD49f, and CD44 as stem cell markers to inhibit stemness; increased keratinocyte differentiation markers, p53, and p21 to induce differentiation and senescence; and decreased cyclooxygenase-2 (COX-2), TNF-α, IL-6, and IL-1B to inhibit NF-κB signaling. As shown in cancer cells, the benzimidazole anthelmintics in animal tumor models inhibited pSTAT3, resulting in decreased tumor growth, increased apoptosis, reduced angiogenesis, and downregulated stemness. They reduced hedgehog signaling, leading to reduced metastasis, inhibited angiogenesis, and increased apoptosis. The benzimidazole anthelmintics decreased G-CSF, GM-CSF, PD-1, and CD11b+Gr1+ myeloid-derived suppressor cell (MDSC) levels, inhibiting tumor growth with a good prognosis.
Benzimidazole anthelmintics decreased cancer markers, such as cancer antigen 125 (CA125), in an ovarian cancer model (65) and prostate-specific antigen (PSA) in a prostate cancer model (71), requiring further studies for other cancer markers. Mebendazole decreased serum alanine aminotransferase (ALT) and alpha-fetoprotein (AFP) in N-nitrosodiethylamine (DEN)-induced hepatocellular carcinoma (51), improving hepatic function, inflammation, and fibrogenesis. Although oxibendazole has increased miR-24a and miR-204 in prostate cancer (71), the effects of benzimidazole anthelmintics on miRNA signature in cancer are still immature, and the benzimidazole anthelmintics may act as specific miRNA regulators to affect cancer prognosis.
Certain formulated-mebendazole and benzimidazole anthelmintics, such as thiabendazole, flubendazole, oxfendazole, and fenbendazole, had no effects on survival in intracranial implantation models of brain cancer cells (92). The intracranial xenograft model of temozolomide-resistant glioblastoma multiforme responded well to mebendazole, but not to albendazole (8). These facts indicate the structure-dependent manner of benzimidazole anthelmintics. Intradermal models of EMT6 mouse mammary tumor cells did not respond to fenbendazole alone or in combination with radiation (28,29). This result indicates the cell-type-specific manner of benzimidazole anthelmintics, identifying sensitive and resistant cancer cells. BSA-albendazole (BSA-ABZ) and nano albumin formulation of albendazole (Nab-ABZ) inhibited tumor weight, ascites fluid, and VEGF in ovarian cancer models, whereas free albendazole had no effects (63). The formulation-dependent effectiveness of albendazole provides a clue to overcoming the low bioavailability of benzimidazole anthelmintics. Furthermore, flubendazole decreased tumor volume in MDA-MB-435 cell-bearing mice after intraperitoneal injection, but not after intratumoral injection (76). This result indicates that the route of benzimidazole anthelmintics administration may be critical for therapeutic application.
Benzimidazole anthelmintics enhanced survival in tumor-bearing animals in combination with temozolomide, elacridar, radiation, sulindac, vincristine, sorafenib, trametinib, and docetaxel. These findings confirm that benzimidazole anthelmintics may be adjuvant therapeutics in combination with conventional chemotherapy. Moreover, albendazole had no synergistic effect with paclitaxel in the ovarian cancer model, indicating that benzimidazole anthelmintics must be used carefully in combination with conventional chemotherapy to maximize therapeutic potential (94). Fenbendazole decreased tumor weight in mammary fat pads of trastuzumab-resistant JIMT-1 cells, providing a possibility of benzimidazole anthelmintics to overcome cancers resistant to conventional chemotherapeutics and recurrent cancers (20).
Despite the safety profile of benzimidazole anthelmintics, some animal studies with mebendazole alone and in combination with vincristine and elacridar showed side effects, such as weight loss and neuropathy. Mebendazole with 100 mg/kg daily administration caused weight loss, whereas the dose of 50 mg/kg did not show adverse effects in C57BL6 mice (8). A combination of mebendazole with vincristine appeared to increase the emergence of neuropathy with rapid weight loss (11). The prolonged treatment course with elacridar and mebendazole increased toxicity in mice, as evidenced by weight loss and mortality (92). These findings require further safety monitoring for the application of benzimidazole anthelmintics alone or in combination with anticancer drugs. We describe the side effects of benzimidazole anthelmintics in the following sections.
ANTITUMORIGENIC EFFECTS OF BENZIMIDAZOLE ANTHELMINTICS IN CLINICAL ASPECTS
The antitumorigenicity of benzimidazole anthelmintics in clinical aspects is summarized (Table 1) (95,96,97,98). Clinical trials of benzimidazole anthelmintics have insufficient data to evaluate theier use in treating cancer. As benzimidazole anthelmintics have been given to patients with refractory solid tumors, metastatic tumors, and failure of conventional chemotherapy, the antitumor effects of benzimidazole anthelmintics on primary tumors are worth clarifying.
Table 1. Antitumorigenicity of benzimidazole anthelmintics in clinical aspects.
| Stage | Cancer type | Purpose | Methods | Results and ongoing | Ref. |
|---|---|---|---|---|---|
| Phase 1 | 36 patients with refractory solid tumors | Maximum-tolerated dose | Albendazole: p.o. on a day 1–14 of a 3-weekly cycle, from 400 mg b.d. to 1,200 mg b.d. | 2,400 mg/day from 1,200 mg b.d. | (95) |
| Decreased plasma VEGF | |||||
| Patients (16%) had a tumor marker response with a fall of at least 50% | |||||
| Adverse effects: myelosuppression, fatigue and mild gastrointestinal upset | |||||
| Case report | 74-year-old man with metastatic colon cancer | Treatment for significant progression in the lungs and abdominal lymph nodes with new partly, poorly defined liver metastases | Mebendazole: 100 mg b.d. for 6 wk | Near complete remission of the metastases in the lungs and lymph nodes and a good partial remission in the liver | (96) |
| 48-year-old man with adrenocortical carcinoma | Treatment for failure or intolerance to conventional treatments with mitotane, 5-fluorouracil, streptozotocin, bevacizumab, and radiation therapy | Mebendazole: 100 mg b.d. for 19 months | Regression of hepatic metastatic lesions and subsequently remained stable for 19 months, but progressed after 24 months | (97) | |
| No clinically adverse effects; quality of life was satisfactory | |||||
| Phase 1 | A patient with hepatocellular carcinoma with metastasis | Evaluation of albendazole | Albendazole: 10 mg/kg/day, p.o. in 2 divided doses for 28 days | Stabilization of the disease, but because of neutropenia, treatment was stopped on day 19 | (98) |
| 8 patients with colorectal cancer with metastasis | Decreased carcinoembryonic antigen (CEA) in 2 patients and stabilized in 3 patients, an initial stabilization (5–10 days) in 2 patients | (98) | |||
| No significant changes in liver and kidney function tests, but neutropenia in 2 patients | |||||
| Phase 1 | 24 high-grade glioma | Mebendazole in newly diagnosed high-grade glioma patients receiving temozolomide | Mebendazole: 500 mg chewable tablets with meals, p.o. 3 times every day on 28-day cycle | Study period: April 4, 2013 to September 2025 | |
| NCT01729260 | Location: The Johns Hopkins Hospital, Baltimore, Maryland, United States | ||||
| NCT02644291 | 21 high-grade glioma | Phase I study of mebendazole therapy for recurrent/progressive pediatric brain tumors | Mebendazole: 500 mg chewable tablets, 3 divided doses with meals | Study period: May 2016 to June 2022 | |
| Location: Johns Hopkins All Children's Hospital | |||||
| Saint Petersburg, Florida, United States | |||||
| Johns Hopkins University School of Medicine | |||||
| Baltimore, Maryland, United States | |||||
| Phases 1/2 | 36 low- and high-grade glioma | A phase I study of mebendazole for the treatment of pediatric gliomas | Mebendazole: 50, 100, and 200 mg/kg/day, p.o. and b.d. for 70 wk for low-grade glioma patients and 48 wk for high-grade glioma patients | Study period: October 22, 2013 to April 2020 | |
| NCT01837862 | Location: Cohen Children's Medical Center of New York, New Hyde Park, New York, United States | ||||
| Phase 2 | 250 patients with malignant disease that is considered untreatable, progressive and fatal | Clinical evaluation of a new form of cancer therapy based on the principles of atavistic metamorphosis (atavistic chemotherapy) | Anti-bacterial, anti-fungal, anti-protozoal agents: anti-cancer properties of albendazole and mebendazole | Study period: July 2011 to December 31, 2023 | |
| NCT02366884 | Location: Dr. Frank Arguello Cancer Clinic | ||||
| San Jose del Cabo, Baja California Sur, Mexico | |||||
| Instituto de Ciencia y Medicina Genomica | |||||
| Torreon, Coahuila, Mexico | |||||
| Phase 3 | 40 patients with stage 4 colorectal cancer | Mebendazole as adjuvant treatment for colon cancer | Mebendazole | Study period: April 1, 2019 to December 2028 | |
| NCT03925662 | Location: Sherief Abd-Elsalam, Cairo, Egypt | ||||
| NCT02201381 | 207 participants with cancer | Study of the safety, tolerability and efficacy of metabolic combination treatments on cancer (METRICS) | Mebendazole: 100 mg, p.o. and u.i.d. for study duration | Study period: May 22, 2017 to May 22, 2022 | |
| Location: Care Oncology Clinic, London, United Kingdom |
b.d., twice daily; p.o., oral administration; u.i.d., once daily dosage.
Thirty-six patients with refractory solid tumors were tolerant of albendazole, even with 2,400 mg/day, resulting in decreased plasma VEGF and tumor markers by 16%. Some patients experienced side effects, such as myelosuppression, fatigue, and mild gastrointestinal upset (95). A patient with metastatic colon cancer responded well to mebendazole, showing nearly complete remission of the metastases in the lungs and lymph nodes and a good partial remission in the liver (96). A patient with adrenocortical carcinoma in failure or intolerance to conventional treatments had taken mebendazole for 19 months. The patient having the regression of hepatic metastatic lesions remained stable for 19 months but progressed after 24 months. No clinically adverse effects were observed, and quality of life was satisfactory (97). Although a patient with metastatic hepatocellular carcinoma remained stable after albendazole doses, treatment was stopped on day 19 due to neutropenia (98). The antitumor effects of albendazole in eight patients with metastatic colorectal cancer were as follows: decreased carcinoembryonic antigen (CEA) in two patients; stabilization in three patients; an initial stabilization (5–10 days) in two patients; no significant changes in liver and kidney function tests in all patients; and neutropenia in 2 patients (98). Based on ClinicalTrials.gov (https://clinicaltrials.gov), we have summarized ongoing clinical trials of albendazole and mebendazole for phases 1, 2, and 3, including low- and high-grade glioma, malignant disease under untreatable, progressive and fatal conditions, and stage 4 colorectal cancer (Table 1). Joe Tippens, a lung cancer survivor, used fenbendazole as a dog de-worming drug to eliminate late-stage small cell lung cancer (https://www.dailymail.co.uk/health/article-6965325/Oklahoma-grandfather-claims-drug-DOGS-cured-cancer-tumor-free.html). Fenbendazole for veterinary use has yet to be established as safe for human use. Albendazole and mebendazole have a priority for clinical trials, rather than other benzimidazole anthelmintics, due to approvals for human use. Clinical trials in the near future will clarify the availabilty of benzimidazole anthelmintics to use as adjuvant and neoadjuvant therapeutics for cancer treatments.
PHARMACOKINETIC PROPERTIES AND SIDE EFFECTS OF BENZIMIDAZOLE ANTHELMINTICS
As benzimidazole anthelmintics have a long history for human and veterinary use, the pharmacokinetic properties and side effects of these anthelmintics are well established based on many studies. Table 2 (5,99,100,101,102,103,104,105,106,107,108,109,110) briefly summarizes the pharmacokinetic properties and side effects of benzimidazole anthelmintics using extracted literature reviews and websites, including www.drugs.com and PARASITIPEDIA.net, emphasizing some issues of these anthelmintics as repurposed drugs for cancer treatment.
Table 2. Pharmacokinetic properties and side effects of benzimidazole anthelmintics.
| Drug | Dosage | Pharmocokinetic properties | Side effects | Ref. |
|---|---|---|---|---|
| Albendazole FDA approval (1996): category C | Human and veterinary use, 400 mg/day, p.o. and b.d. for 1 to 6 months in Echinococcus infection | Absorption: poor solubility and absorption (<5% in humans and 50% in cattle); increase up to 5 times when administered with a fatty meal | Common: headaches and hepatotoxicity with elevated liver enzymes | (5,99,100,101,102) |
| Distribution: widely distributed throughout the body including urine, bile, liver, cyst wall, cyst fluid, and CSF | Few: abdominal pain, nausea, vomiting, fever, and hypersensitivity reaction, such as hives and pruritus | |||
| Metabolism: Hepatic extensive first-pass effect; rapid sulfoxidation to active metabolite albendazole sulfoxide and finally inactive albendazole sulfone, hydrolysis, and oxidation | Rare: alopecia, telogen effluvium | |||
| Excretion: urine (<1% as active metabolite) and feces, hepatic clearance = 18.2 ml/min/kg | Other: leukopenia, anemia, thrombocytopenia, pancytopenia | |||
| Time to peak = 2–5 h for albendazole sulfoxide in serum | Avoid during pregnancy: teratogenic effects in the offspring of rats, but pregnant patients who received albendazole did not show an increased risk of teratogenicity | |||
| t1/2 = 8–12 h for albendazole sulfoxide | No risk of aneuploidy for conventional therapeutic use of albendazole in human | |||
| Protein binding = 70% | ||||
| Mebendazole approved in the United States in 1974 | Human and veterinary use | Absorption: poor solubility and absorption, 5%–10% in humans and 1%–2% after a high dose, enhanced by eating high-fat meals distribution: Vd = 1–2 L/kg | Anthelmintic spectrum and adverse effect profile of mebendazole is almost identical to albendazole | (5,101,103,104) |
| Some experts recommend: 200 to 400 mg, p.o. and t.i.d. for 3 days, then 400 to 500 mg, p.o. and t.i.d. for 10 days for trichinosis | Metabolism: extensively hepatic first pass metabolism involving keto-reduction and decarbamylation, followed by conjugation | Relatively non-toxic, well-tolerated | ||
| Excretion: primarily feces (as unchanged drug and primary metabolite) and urine (<2%) | Side effects are uncommon with rare cases, such as gastrointestinal upset, fever, diarrhea, abdominal pain, discomfort, flatulence, diarrhea, hypersensitivity reactions, such as rash, urticaria, and angioedema | |||
| t1/2 = 3–6 h | High doses may induce anemia and hepatotoxicity with rare instances of neutropenia, marrow aplasia, alopecia | |||
| Protein Binding = 90%–95% | Rodents showed fetal toxicity and teratogenicity at high doses, but not other species including rabbits, horses, sheep, and swine | |||
| Pass through the blood–brain barrier | Contraindicated for pregnancy | |||
| Aneuploidy in in vitro and in vivo experiments but not considered to be a risk to humans receiving conventional therapy | ||||
| Fenbendazole | Veterinary use | Metabolism: extensively hepatic first pass to the active metabolite fenbendazole sulfoxide (active form) and finally fenbendazole sulfone | A nontoxic drug in rodents: LD50 exceeds 10 g/kg (a dose 1,000 times the therapeutic level) | (105,106) |
| Excretion: primarily feces and urine | Lifetime studies in rats: lack of carcinogenesis, no maternal and reproductive toxicity | |||
| Morphologic changes of hepatocellular hypertrophy and hyperplasia in rats | ||||
| No observed adverse effect in mice | ||||
| Myelosuppression in dogs and birds, but not in rodents | ||||
| Ricobendazole (albendazole sulfoxide) | Absorption: poor bioavailability and enhanced hydrosolubility | Ricobendazole is a key metabolite of albendazole; may have side effects similar to albendazole | (5,105) | |
| Vd = 0.67–1.2 L/kg in cattle and sheep | ||||
| Metabolism: absorbed oxfendazole is partly and reversibly reduced to albendazole both in the liver and in the rumen | ||||
| Excretion: feces and urine | ||||
| t1/2 = 8–12 h in man | ||||
| Protein binding = 70% | ||||
| Crosses the blood–brain barrier | ||||
| Ricobendazole enantiomers have a species difference in pharmacokinetic profiles | ||||
| Oxfendazole (fenbendazole sulfoxide) | Veterinary use | Enhanced hydrosolubility | Well-tolerated in most species | (105,107) |
| Distribution throughout the body | Main symptoms of intoxication after high oral doses: loss of appetite, diarrhea, fever, cramps, nausea, vomit and convulsions | |||
| Metabolism: absorbed oxfendazole is partly and reversibly reduced to fenbendazole both in the liver and in the rumen | Hepatic and epicardial hemorraghe can also happen | |||
| Excretion: 80% through bile and feces in ruminants | Mutagenic effects, embryotoxicity, teratogenicity in mice | |||
| t1/2 = 8–12 h in human (NCT03035760, NCT02234570) | ||||
| Oxfendazole enantiomers have a species difference in pharmacokinetic profiles | ||||
| Flubendazole | Veterinary use | Absorption: poor bioavailability | Well-tolerated without adverse effects | (108) |
| Metabolism: extensive first pass via carbamate hydrolysis and ketone reduction to inactive metabolites | Not be used in pregnant or lactating queens, nor in puppies younger than 1 year | |||
| Excretion: more than 80% of p.o. dose in feces and only very small amounts of unchanged drug (less than 0.1%) in the urine | ||||
| t1/2 in tissues = 1–2 days | ||||
| Oxibendazole | Veterinary use | Absorption: poor bioavailability but increased bioavailabiblity in sheep and goats splitting the therapeutic dose during 3 consecutive days (each day 1/3 of the recommended dose) | Well-tolerated in most species | (109) |
| Metabolism: little information but expected broken down in the liver to metabolites without anthelmintic activity | No acute toxicity with single oral doses in mice (4–32 g/kg of body weight), sheep (230–600 mg/kg), and cattle (600 mg/kg) | |||
| No subacute toxicity with multiple doses for 5 days in cattle (30–75 mg/kg/day) and sheep (10–50 mg/kg/day) | ||||
| No chronic effects with 3–30 mg/kg for 98 days in rats and dogs | ||||
| No teratogenicity in mice, rats, sheep at 30 mg/kg and cattle at 75 mg/kg during pregnancy | ||||
| Main symptoms after high oral doses: vomiting, depression, trembling | ||||
| Parbendazole | Veterinary use | Peak blood levels = 6–8 h after administration | Pregnant animals are contraindicated: teratogenicity is largely skeletal | (110) |
| Laxation (soft dung/diarrhea), anorexia, listlessness |
LD50, lethal dose, 50%; p.o., oral administration; t1/2, half-life time; t.i.d., three times a day; Vd, volume of distribution.
The general pharmacokinetic properties of benzimidazole anthelmintics are as follows: poor absorption; wide distribution in the body; extensive hepatic metabolism; and excretion via feces and urine (Table 2). The absorption of benzimidazole anthelmintics is poor, resulting in low bioavailability. The oral absorption of albendazole is about 20%–30% in mice and rats, about 50% in cattle, and about 1%–<5% in humans (5). The low water solubility and low intestinal absorption rate of benzimidazole anthelmintics may make it difficult to achieve effective concentrations in the systemic circulation to treat cancer in humans. A fatty meal enhances absorption up to 5-fold in humans and animals. Micro-emulsifying delivery systems and increased lipid solubility in dosing benzimidazole anthelmintics help to maximize the therapeutic efficacy. The bioavailability of albendazole in fasted calves was markedly greater than that in calves fed ad libitum (111), indicating better bioavailability in fasting conditions. Various formulation strategies attempt to circumvent the low solubility and poor bioavailability of benzimidazole anthelmintics through the use of organic co-solvents, liposomes, surfactants, nanoparticles, microspheres, and cyclodextrins. Nanoformulations of albendazole and conjugated albendazole with nano-carriers improve solubility and increase drug delivery to tumor cells (112). This increased bioavailability will enhance the antitumor potential of benzimidazole anthelmintics, which are generally safe and well-tolerated in human and veterinary use. The common side effects of benzimidazole anthelmintics are as follows: headaches and hepatotoxicity in common; leukopenia and anemia occasionally; and issues in the gastrointestinal tract, such as laxation and anorexia (Table 2). Although albendazole has hepatotoxicity, liver function in patients recovered rapidly on the withdrawal of albendazole (113). Benzimidazole anthelmintics must be used with caution in patients with reduced liver function and with liver cirrhosis and liver cancer. Although albendazole stabilized cancer conditions in a patient with metastatic hepatocellular carcinoma, the treatment was stopped due to neutropenia (98). Albendazole is known to be a teratogen and a fetal toxicant in rodents (114), indicating contraindication during pregnancy. Netobimin, a prodrug of albendazole, increased resorption and skeletal malformations and decreased fetal body weight in pregnant rats (115). However, it does not appear to induce a teratogenic action in humans, even in women dosed during pregnancy (5). The differential toxicity of benzimidazole anthelmintics among species indicates species-specific effects. Although fenbendazole may be considered a nontoxic drug in rodents (106), benzimidazole anthelmintics for veterinary use have not led to enough evidence of safety for human use, requiring further studies.
SUMMARY
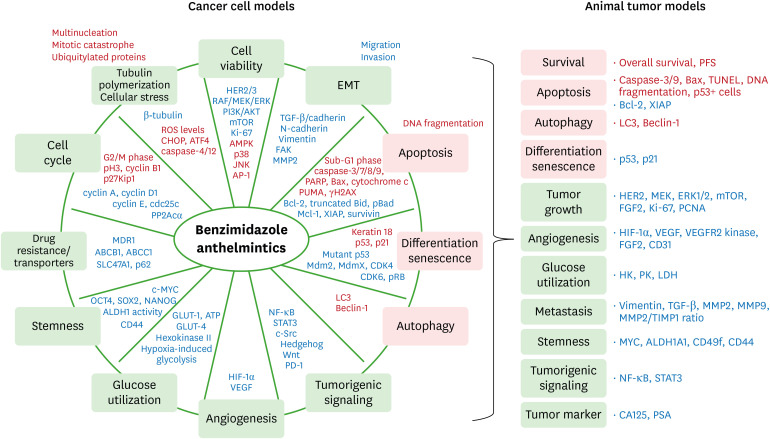
Benzimidazole anthelmintics induce minimal cytotoxicity in normal cells but high cytotoxicity in tumor cells, exerting a cancer cell-specific selectivity. The antitumor effects of benzimidazole anthelmintics are exerted through various biological actions, such as inhibited cell viability, migration, and invasion, reduced colony formation, disrupted tubulin polymerization, induced apoptosis and autophagy, G2/M cell cycle arrest, induced differentiation and senescence, reduced angiogenesis, inhibited drug resistance and transporters, and impaired glucose utilization. Specific signaling pathways related to these biological actions of these compounds support the antitumorigenicity of benzimidazole anthelmintics (Fig. 2). In addition to in vitro cancer cell lines, benzimidazole anthelmintics have shown antitumor effects in in vivo animal tumor models, such as prolonged overall survival and progression-free survival, inhibited tumor growth, reduced vessel formation, and reduced metastasis, all of which share signaling pathways shown in cancer cell models (Fig. 2). Almost all animals tolerated benzimidazole anthelmintics without significant side effects. These benzimidazole anthelmintics have demonstrated the synergistic and enhanced antitumorigenicity in combination with conventional chemotherapeutics and radiation, appearing to be a promising candidate for adjuvant and neoadjuvant therapy with low cost. Some cancer cells have tendency for resistence to benzimidazole anthelmintics, requiring mechanistic studies to explain the cause. Clinical trials for the antitumor effects of benzimidazole anthelmintics have insufficient data to demonstrate the availability to treat human cancers. Despite the limited data, some patients with metastatic late-stage cancers responded well to albendazole and mebendazole showing reduced tumor markers and metastasis and stabilized disease. Ongoing clinical trials will provide insight to benzimidazole anthelmintics for primary and metastatic cancer treatments in the near future. Although benzimidazole anthelmintics have a broad safety spectrum, the poor bioavailability of these anthelmintics is the main hindrance, requiring better formulation strategies and fatty meals to increase the solubility. Increased bioavailability of benzimidazole anthelmintics may improve antitumorigenesis and prolong overall survival. Although benzimidazole anthelmintics for veterinary care have well-known safety, the safety has not been established for human use, requiring further studies.
Figure 2. Summarized schemes for the antitumorigenicity of benzimidazole anthelmintics in cancer. Light green boxes, inhibited biological aspects; pink boxes, induced biological aspects; red letters, upregulated signalings and reactions; blue letters, downregulated signalings and reactions. See Tables 1 and 2 for genes and proteins.
ACKNOWLEDGEMENTS
This research was supported, in whole or in part, by the National Institutes of Health (NIH) under the following grants: NCI SC1CA200519 (D.S.) and U54CA163069 (S.E.A. and D.S.); RCMI 5U54AMD007586 (S.E.A.); and R01ES024756 (E.L.). Research contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Editing services were provided by the Meharry Office of Scientific Editing and Publications (NIH S21MD000104).
Abbreviations
- ABZ
albendazole
- AFP
alpha-fetoprotein
- AKT
protein kinase B
- ALDH1
aldehyde dehydrogenase 1
- ALT
alanine aminotransferase
- AMPK
AMP-activated protein kinase
- AP-1
activator protein 1
- ATF
activating transcription factor
- BAD
Bcl-2 associated agonist of cell death
- Bax
Bcl-2-associated X protein
- Bcl-2
B-cell lymphoma 2
- BSA-ABZ
BSA-albendazole
- CA
cancer antigen
- CEA
carcinoembryonic antigen
- CHOP
C/EBP homologous protein
- c-MYC
a human homology with the viral gene v-myc
- COX-2
cyclooxygenase-2
- DEN
N-nitrosodiethylamine
- EMT
epithelial-to-mesenchymal transition
- ER
endoplasmic reticulum
- FDA
Food and Drug Administration
- FGF
fibroblast growth factor
- GLUT
glucose transporter
- HCC
hepatocellular carcinoma
- HER
human epidermal growth factor receptor
- HIF
hypoxia-inducible factor
- HK
hexokinase
- i.p.
intraperitoneal injection
- i.t.
intratumoral injection
- i.v.
intravenous injection
- IC50
half maximal inhibitory concentration
- IκBα
nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha
- JNK
c-Jun N-terminal kinases
- KRAS
Kirsten rat sarcoma viral oncogene homolog
- LC3
microtubule-associated protein 1 light chain 3
- Mcl-1
myeloid cell factor-1
- Mdm2
mouse double minute 2 homolog
- MdmX
mouse double minute 4
- MDSC
myeloid-derived suppressor cell
- miR
microRNA
- MMP
matrix metalloproteinase
- Nab-ABZ
nano albumin formulation of albendazole
- p
phosphorylated
- p.o.
oral administration
- PARP
poly(ADP-ribose) polymerase
- PCNA
proliferating cell nuclear antigen
- PFS
progression-free survival
- pH3
phospho-Histone H3
- PK
pyruvate kinase
- PP2A
protein phosphatase 2A
- PSA
prostate-specific antigen
- PUMA
p53 upregulated modulator of apoptosis
- RAF
rapidly accelerated fibrosarcoma (a family of three serine/threonine-specific protein kinases)
- RB
retinoblastoma protein
- s.c.
subcutaneous injection
- SIRT
sirtuin
- SOX2
SRY (sex determining region Y)-box 2
- SRF
serum response factor
- TCF4
T-cell factor-4
- TIMP
tissue inhibitor of metalloproteinase
- TMZ
temozolomide
- TNIK
TRAF2- and NCK-interacting kinase
- TTP
tristetraprolin
- TUNEL
transferase dUTP nick end labeling
- VEGFR2
vascular endothelial growth factor receptor 2
- XIAP
X-linked inhibitor of apoptosis protein
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Son DS.
- Data curation: Son DS, Lee ES, Adunyah SE.
- Formal analysis: Son DS, Lee ES.
- Funding acquisition: Son DS, Lee ES, Adunyah SE.
- Investigation: Son DS.
- Methodology: Son DS.
- Supervision: Son DS.
- Validation: Son DS, Lee ES, Adunyah SE.
- Visualization: Son DS.
- Writing - original draft: Son DS.
- Writing - review & editing: Son DS, Lee ES, Adunyah SE.
SUPPLEMENTARY MATERIALS
Antitumorigenicity of benzimidazole anthelmintics on cancer cell lines
Antitumorigenicity of benzimidazole anthelmintics in animal tumor models
References
- 1.Furtado LFV, de Paiva Bello ACP, Rabelo ÉML. Benzimidazole resistance in helminths: from problem to diagnosis. Acta Trop. 2016;162:95–102. doi: 10.1016/j.actatropica.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 2.Friedman PA, Platzer EG. Interaction of anthelmintic benzimidazoles with Ascaris suum embryonic tubulin. Biochim Biophys Acta. 1980;630:271–278. doi: 10.1016/0304-4165(80)90431-6. [DOI] [PubMed] [Google Scholar]
- 3.Cleeland CS, Allen JD, Roberts SA, Brell JM, Giralt SA, Khakoo AY, Kirch RA, Kwitkowski VE, Liao Z, Skillings J. Reducing the toxicity of cancer therapy: recognizing needs, taking action. Nat Rev Clin Oncol. 2012;9:471–478. doi: 10.1038/nrclinonc.2012.99. [DOI] [PubMed] [Google Scholar]
- 4.Tang Y, Liang J, Wu A, Chen Y, Zhao P, Lin T, Zhang M, Xu Q, Wang J, Huang Y. Co-delivery of trichosanthin and albendazole by nano-self-assembly for overcoming tumor multidrug-resistance and metastasis. ACS Appl Mater Interfaces. 2017;9:26648–26664. doi: 10.1021/acsami.7b05292. [DOI] [PubMed] [Google Scholar]
- 5.Dayan AD. Albendazole, mebendazole and praziquantel. Review of non-clinical toxicity and pharmacokinetics. Acta Trop. 2003;86:141–159. doi: 10.1016/s0001-706x(03)00031-7. [DOI] [PubMed] [Google Scholar]
- 6.Lai SR, Castello SA, Robinson AC, Koehler JW. In vitro anti-tubulin effects of mebendazole and fenbendazole on canine glioma cells. Vet Comp Oncol. 2017;15:1445–1454. doi: 10.1111/vco.12288. [DOI] [PubMed] [Google Scholar]
- 7.Marslin G, Siram K, Liu X, Khandelwal VKM, Xiaolei S, Xiang W, Franklin G. Solid lipid nanoparticles of albendazole for enhancing cellular uptake and cytotoxicity against U-87 MG glioma cell lines. Molecules. 2017;22:2040. doi: 10.3390/molecules22112040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bai RY, Staedtke V, Aprhys CM, Gallia GL, Riggins GJ. Antiparasitic mebendazole shows survival benefit in 2 preclinical models of glioblastoma multiforme. Neuro Oncol. 2011;13:974–982. doi: 10.1093/neuonc/nor077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kipper FC, Silva AO, Marc AL, Confortin G, Junqueira AV, Neto EP, Lenz G. Vinblastine and antihelmintic mebendazole potentiate temozolomide in resistant gliomas. Invest New Drugs. 2018;36:323–331. doi: 10.1007/s10637-017-0503-7. [DOI] [PubMed] [Google Scholar]
- 10.Markowitz D, Ha G, Ruggieri R, Symons M. Microtubule-targeting agents can sensitize cancer cells to ionizing radiation by an interphase-based mechanism. Onco Targets Ther. 2017;10:5633–5642. doi: 10.2147/OTT.S143096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Witt M, Gamble A, Hanson D, Markowitz D, Powell C, Al Dimassi S, Atlas M, Boockvar J, Ruggieri R, Symons M. Repurposing mebendazole as a replacement for vincristine for the treatment of brain tumors. Mol Med. 2017;23:50–56. doi: 10.2119/molmed.2017.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larsen AR, Bai RY, Chung JH, Borodovsky A, Rudin CM, Riggins GJ, Bunz F. Repurposing the antihelmintic mebendazole as a hedgehog inhibitor. Mol Cancer Ther. 2015;14:3–13. doi: 10.1158/1535-7163.MCT-14-0755-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skibinski CG, Williamson T, Riggins GJ. Mebendazole and radiation in combination increase survival through anticancer mechanisms in an intracranial rodent model of malignant meningioma. J Neurooncol. 2018;140:529–538. doi: 10.1007/s11060-018-03009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michaelis M, Agha B, Rothweiler F, Löschmann N, Voges Y, Mittelbronn M, Starzetz T, Harter PN, Abhari BA, Fulda S, et al. Identification of flubendazole as potential anti-neuroblastoma compound in a large cell line screen. Sci Rep. 2015;5:8202. doi: 10.1038/srep08202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H, Sun H, Zhang B, Liu S, Deng S, Weng Z, Zuo B, Yang J, He Y. 18F-FDG PET imaging for monitoring the early anti-tumor effect of albendazole on triple-negative breast cancer. Breast Cancer. 2020;27:372–380. doi: 10.1007/s12282-019-01027-5. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Bochkur Dratver M, Yazal T, Dong K, Nguyen A, Yu G, Dao A, Bochkur Dratver M, Duhachek-Muggy S, Bhat K, et al. Mebendazole potentiates radiation therapy in triple-negative breast cancer. Int J Radiat Oncol Biol Phys. 2019;103:195–207. doi: 10.1016/j.ijrobp.2018.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh E, Kim YJ, An H, Sung D, Cho TM, Farrand L, Jang S, Seo JH, Kim JY. Flubendazole elicits anti-metastatic effects in triple-negative breast cancer via STAT3 inhibition. Int J Cancer. 2018;143:1978–1993. doi: 10.1002/ijc.31585. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Guo M, Li J, Zheng Y, Zhang S, Xie T, Liu B. Systems biology-based discovery of a potential Atg4B agonist (Flubendazole) that induces autophagy in breast cancer. Mol Biosyst. 2015;11:2860–2866. doi: 10.1039/c5mb00466g. [DOI] [PubMed] [Google Scholar]
- 19.Hou ZJ, Luo X, Zhang W, Peng F, Cui B, Wu SJ, Zheng FM, Xu J, Xu LZ, Long ZJ, et al. Flubendazole, FDA-approved anthelmintic, targets breast cancer stem-like cells. Oncotarget. 2015;6:6326–6340. doi: 10.18632/oncotarget.3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim YJ, Sung D, Oh E, Cho Y, Cho TM, Farrand L, Seo JH, Kim JY. Flubendazole overcomes trastuzumab resistance by targeting cancer stem-like properties and HER2 signaling in HER2-positive breast cancer. Cancer Lett. 2018;412:118–130. doi: 10.1016/j.canlet.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 21.Coyne CP, Jones T, Bear R. Gemcitabine-(C4-amide)-[anti-HER2/neu] anti-neoplastic cytotoxicity in dual combination with mebendazole against chemotherapeutic-resistant mammary adenocarcinoma. J Clin Exp Oncol. 2013;2:1000109. [PMC free article] [PubMed] [Google Scholar]
- 22.Belaz KR, Denadai M, Almeida AP, Lima RT, Vasconcelos MH, Pinto MM, Cass QB, Oliveira RV. Enantiomeric resolution of albendazole sulfoxide by semipreparative HPLC and in vitro study of growth inhibitory effects on human cancer cell lines. J Pharm Biomed Anal. 2012;66:100–108. doi: 10.1016/j.jpba.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Mrkvová Z, Uldrijan S, Pombinho A, Bartůněk P, Slaninová I. Benzimidazoles downregulate Mdm2 and MdmX and activate p53 in MdmX overexpressing tumor cells. Molecules. 2019;24:2152. doi: 10.3390/molecules24112152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zanganeh S, Khosravi S, Namdar N, Amiri MH, Gharooni M, Abdolahad M. Electrochemical approach for monitoring the effect of anti tubulin drugs on breast cancer cells based on silicon nanograss electrodes. Anal Chim Acta. 2016;938:72–81. doi: 10.1016/j.aca.2016.07.042. [DOI] [PubMed] [Google Scholar]
- 25.Shashaani H, Faramarzpour M, Hassanpour M, Namdar N, Alikhani A, Abdolahad M. Silicon nanowire based biosensing platform for electrochemical sensing of Mebendazole drug activity on breast cancer cells. Biosens Bioelectron. 2016;85:363–370. doi: 10.1016/j.bios.2016.04.081. [DOI] [PubMed] [Google Scholar]
- 26.Taranejoo S, Janmaleki M, Pachenari M, Seyedpour SM, Chandrasekaran R, Cheng W, Hourigan K. Dual effect of F-actin targeted carrier combined with antimitotic drug on aggressive colorectal cancer cytoskeleton: allying dissimilar cell cytoskeleton disrupting mechanisms. Int J Pharm. 2016;513:464–472. doi: 10.1016/j.ijpharm.2016.09.056. [DOI] [PubMed] [Google Scholar]
- 27.Priotti J, Baglioni MV, García A, Rico MJ, Leonardi D, Lamas MC, Menacho Márquez M. Repositioning of anti-parasitic drugs in cyclodextrin inclusion complexes for treatment of triple-negative breast cancer. AAPS PharmSciTech. 2018;19:3734–3741. doi: 10.1208/s12249-018-1169-y. [DOI] [PubMed] [Google Scholar]
- 28.Duan Q, Liu Y, Booth CJ, Rockwell S. Use of fenbendazole-containing therapeutic diets for mice in experimental cancer therapy studies. J Am Assoc Lab Anim Sci. 2012;51:224–230. [PMC free article] [PubMed] [Google Scholar]
- 29.Duan Q, Liu Y, Rockwell S. Fenbendazole as a potential anticancer drug. Anticancer Res. 2013;33:355–362. [PMC free article] [PubMed] [Google Scholar]
- 30.Spagnuolo PA, Hu J, Hurren R, Wang X, Gronda M, Sukhai MA, Di Meo A, Boss J, Ashali I, Beheshti Zavareh R, et al. The antihelmintic flubendazole inhibits microtubule function through a mechanism distinct from Vinca alkaloids and displays preclinical activity in leukemia and myeloma. Blood. 2010;115:4824–4833. doi: 10.1182/blood-2009-09-243055. [DOI] [PubMed] [Google Scholar]
- 31.Králová V, Hanušová V, Staňková P, Knoppová K, Čáňová K, Skálová L. Antiproliferative effect of benzimidazole anthelmintics albendazole, ricobendazole, and flubendazole in intestinal cancer cell lines. Anticancer Drugs. 2013;24:911–919. doi: 10.1097/CAD.0b013e3283648c69. [DOI] [PubMed] [Google Scholar]
- 32.Hanušová V, Skálová L, Králová V, Matoušková P. The effect of flubendazole on adhesion and migration in SW480 and SW620 colon cancer cells. Anticancer Agents Med Chem. 2018;18:837–846. doi: 10.2174/1871520618666171213141911. [DOI] [PubMed] [Google Scholar]
- 33.Králová V, Hanušová V, Rudolf E, Čáňová K, Skálová L. Flubendazole induces mitotic catastrophe and senescence in colon cancer cells in vitro . J Pharm Pharmacol. 2016;68:208–218. doi: 10.1111/jphp.12503. [DOI] [PubMed] [Google Scholar]
- 34.Lin S, Yang L, Yao Y, Xu L, Xiang Y, Zhao H, Wang L, Zuo Z, Huang X, Zhao C. Flubendazole demonstrates valid antitumor effects by inhibiting STAT3 and activating autophagy. J Exp Clin Cancer Res. 2019;38:293. doi: 10.1186/s13046-019-1303-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williamson T, Bai RY, Staedtke V, Huso D, Riggins GJ. Mebendazole and a non-steroidal anti-inflammatory combine to reduce tumor initiation in a colon cancer preclinical model. Oncotarget. 2016;7:68571–68584. doi: 10.18632/oncotarget.11851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ehteda A, Galettis P, Pillai K, Morris DL. Combination of albendazole and 2-methoxyestradiol significantly improves the survival of HCT-116 tumor-bearing nude mice. BMC Cancer. 2013;13:86. doi: 10.1186/1471-2407-13-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ehteda A, Galettis P, Chu SW, Pillai K, Morris DL. Complexation of albendazole with hydroxypropyl-β-cyclodextrin significantly improves its pharmacokinetic profile, cell cytotoxicity and antitumor efficacy in nude mice. Anticancer Res. 2012;32:3659–3666. [PubMed] [Google Scholar]
- 38.Dogra N, Mukhopadhyay T. Impairment of the ubiquitin-proteasome pathway by methyl N-(6-phenylsulfanyl-1H-benzimidazol-2-yl)carbamate leads to a potent cytotoxic effect in tumor cells: a novel antiproliferative agent with a potential therapeutic implication. J Biol Chem. 2012;287:30625–30640. doi: 10.1074/jbc.M111.324228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pourgholami MH, Akhter J, Wang L, Lu Y, Morris DL. Antitumor activity of albendazole against the human colorectal cancer cell line HT-29: in vitro and in a xenograft model of peritoneal carcinomatosis. Cancer Chemother Pharmacol. 2005;55:425–432. doi: 10.1007/s00280-004-0927-6. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, Zhao J, Gao X, Pei D, Gao C. Anthelmintic drug albendazole arrests human gastric cancer cells at the mitotic phase and induces apoptosis. Exp Ther Med. 2017;13:595–603. doi: 10.3892/etm.2016.3992. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Pinto LC, Soares BM, Pinheiro JJ, Riggins GJ, Assumpção PP, Burbano RM, Montenegro RC. The anthelmintic drug mebendazole inhibits growth, migration and invasion in gastric cancer cell model. Toxicol In Vitro. 2015;29:2038–2044. doi: 10.1016/j.tiv.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 42.Pinto LC, Mesquita FP, Soares BM, da Silva EL, Puty B, de Oliveira EHC, Burbano RR, Montenegro RC. Mebendazole induces apoptosis via C-MYC inactivation in malignant ascites cell line (AGP01) Toxicol In Vitro. 2019;60:305–312. doi: 10.1016/j.tiv.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 43.Celestino Pinto L, de Fátima Aquino Moreira-Nunes C, Soares BM, Burbano RMR, de Lemos JAR, Montenegro RC. Mebendazole, an antiparasitic drug, inhibits drug transporters expression in preclinical model of gastric peritoneal carcinomatosis. Toxicol In Vitro. 2017;43:87–91. doi: 10.1016/j.tiv.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 44.Ghasemi F, Black M, Vizeacoumar F, Pinto N, Ruicci KM, Le CC, Lowerison MR, Leong HS, Yoo J, Fung K, et al. Repurposing albendazole: new potential as a chemotherapeutic agent with preferential activity against HPV-negative head and neck squamous cell cancer. Oncotarget. 2017;8:71512–71519. doi: 10.18632/oncotarget.17292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang F, Li Y, Zhang H, Huang E, Gao L, Luo W, Wei Q, Fan J, Song D, Liao J, et al. Anthelmintic mebendazole enhances cisplatin's effect on suppressing cell proliferation and promotes differentiation of head and neck squamous cell carcinoma (HNSCC) Oncotarget. 2017;8:12968–12982. doi: 10.18632/oncotarget.14673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang LJ, Lee YC, Huang CH, Shi YJ, Chen YJ, Pei SN, Chou YW, Chang LS. Non-mitotic effect of albendazole triggers apoptosis of human leukemia cells via SIRT3/ROS/p38 MAPK/TTP axis-mediated TNF-α upregulation. Biochem Pharmacol. 2019;162:154–168. doi: 10.1016/j.bcp.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 47.Li Y, Thomas D, Deutzmann A, Majeti R, Felsher DW, Dill DL. Mebendazole for differentiation therapy of acute myeloid leukemia identified by a lineage maturation index. Sci Rep. 2019;9:16775. doi: 10.1038/s41598-019-53290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blom K, Senkowski W, Jarvius M, Berglund M, Rubin J, Lenhammar L, Parrow V, Andersson C, Loskog A, Fryknäs M, et al. The anticancer effect of mebendazole may be due to M1 monocyte/macrophage activation via ERK1/2 and TLR8-dependent inflammasome activation. Immunopharmacol Immunotoxicol. 2017;39:199–210. doi: 10.1080/08923973.2017.1320671. [DOI] [PubMed] [Google Scholar]
- 49.Khalilzadeh A, Wangoo KT, Morris DL, Pourgholami MH. Epothilone-paclitaxel resistant leukemic cells CEM/dEpoB300 are sensitive to albendazole: Involvement of apoptotic pathways. Biochem Pharmacol. 2007;74:407–414. doi: 10.1016/j.bcp.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 50.Kang BS, Lee SE, Ng CL, Kim JK, Park JS. Exploring the preparation of albendazole-loaded chitosan-tripolyphosphate nanoparticles. Materials (Basel) 2015;8:486–498. doi: 10.3390/ma8020486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Younis NS, Ghanim AMH, Saber S. Mebendazole augments sensitivity to sorafenib by targeting MAPK and BCL-2 signalling in n-nitrosodiethylamine-induced murine hepatocellular carcinoma. Sci Rep. 2019;9:19095. doi: 10.1038/s41598-019-55666-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pourgholami MH, Woon L, Almajd R, Akhter J, Bowery P, Morris DL. In vitro and in vivo suppression of growth of hepatocellular carcinoma cells by albendazole. Cancer Lett. 2001;165:43–49. doi: 10.1016/s0304-3835(01)00382-2. [DOI] [PubMed] [Google Scholar]
- 53.Shimomura I, Yokoi A, Kohama I, Kumazaki M, Tada Y, Tatsumi K, Ochiya T, Yamamoto Y. Drug library screen reveals benzimidazole derivatives as selective cytotoxic agents for KRAS-mutant lung cancer. Cancer Lett. 2019;451:11–22. doi: 10.1016/j.canlet.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 54.Zhou F, Du J, Wang J. Albendazole inhibits HIF-1α-dependent glycolysis and VEGF expression in non-small cell lung cancer cells. Mol Cell Biochem. 2017;428:171–178. doi: 10.1007/s11010-016-2927-3. [DOI] [PubMed] [Google Scholar]
- 55.Dogra N, Kumar A, Mukhopadhyay T. Fenbendazole acts as a moderate microtubule destabilizing agent and causes cancer cell death by modulating multiple cellular pathways. Sci Rep. 2018;8:11926. doi: 10.1038/s41598-018-30158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sasaki J, Ramesh R, Chada S, Gomyo Y, Roth JA, Mukhopadhyay T. The anthelmintic drug mebendazole induces mitotic arrest and apoptosis by depolymerizing tubulin in non-small cell lung cancer cells. Mol Cancer Ther. 2002;1:1201–1209. [PubMed] [Google Scholar]
- 57.Mukhopadhyay T, Sasaki J, Ramesh R, Roth JA. Mebendazole elicits a potent antitumor effect on human cancer cell lines both in vitro and in vivo . Clin Cancer Res. 2002;8:2963–2969. [PubMed] [Google Scholar]
- 58.Xu D, Tian W, Jiang C, Huang Z, Zheng S. The anthelmintic agent oxfendazole inhibits cell growth in non‑small cell lung cancer by suppressing c-Src activation. Mol Med Rep. 2019;19:2921–2926. doi: 10.3892/mmr.2019.9897. [DOI] [PubMed] [Google Scholar]
- 59.Patel K, Doudican NA, Schiff PB, Orlow SJ. Albendazole sensitizes cancer cells to ionizing radiation. Radiat Oncol. 2011;6:160. doi: 10.1186/1748-717X-6-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kralova V, Hanušová V, Caltová K, Špaček P, Hochmalová M, Skálová L, Rudolf E. Flubendazole and mebendazole impair migration and epithelial to mesenchymal transition in oral cell lines. Chem Biol Interact. 2018;293:124–132. doi: 10.1016/j.cbi.2018.07.026. [DOI] [PubMed] [Google Scholar]
- 61.Pillai K, Akhter J, Morris DL. Super aqueous solubility of albendazole in β-cyclodextrin for parenteral application in cancer therapy. J Cancer. 2017;8:913–923. doi: 10.7150/jca.17301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pourgholami MH, Wangoo KT, Morris DL. Albendazole-cyclodextrin complex: enhanced cytotoxicity in ovarian cancer cells. Anticancer Res. 2008;28:2775–2779. [PubMed] [Google Scholar]
- 63.Noorani L, Stenzel M, Liang R, Pourgholami MH, Morris DL. Albumin nanoparticles increase the anticancer efficacy of albendazole in ovarian cancer xenograft model. J Nanobiotechnology. 2015;13:25. doi: 10.1186/s12951-015-0082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pourgholami MH, Cai ZY, Badar S, Wangoo K, Poruchynsky MS, Morris DL. Potent inhibition of tumoral hypoxia-inducible factor 1alpha by albendazole. BMC Cancer. 2010;10:143. doi: 10.1186/1471-2407-10-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pourgholami MH, Yan Cai Z, Lu Y, Wang L, Morris DL. Albendazole: a potent inhibitor of vascular endothelial growth factor and malignant ascites formation in OVCAR-3 tumor-bearing nude mice. Clin Cancer Res. 2006;12:1928–1935. doi: 10.1158/1078-0432.CCR-05-1181. [DOI] [PubMed] [Google Scholar]
- 66.Hettiarachchi G, Samanta SK, Falcinelli S, Zhang B, Moncelet D, Isaacs L, Briken V. Acyclic cucurbit[n]uril-type molecular container enables systemic delivery of effective doses of albendazole for treatment of SK-OV-3 xenograft tumors. Mol Pharm. 2016;13:809–818. doi: 10.1021/acs.molpharmaceut.5b00723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chu SW, Badar S, Morris DL, Pourgholami MH. Potent inhibition of tubulin polymerisation and proliferation of paclitaxel-resistant 1A9PTX22 human ovarian cancer cells by albendazole. Anticancer Res. 2009;29:3791–3796. [PubMed] [Google Scholar]
- 68.Florio R, Veschi S, di Giacomo V, Pagotto S, Carradori S, Verginelli F, Cirilli R, Casulli A, Grassadonia A, Tinari N, et al. The benzimidazole-based anthelmintic parbendazole: a repurposed drug candidate that synergizes with gemcitabine in pancreatic cancer. Cancers (Basel) 2019;11:11. doi: 10.3390/cancers11122042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen H, Weng Z, Xu C. Albendazole suppresses cell proliferation and migration and induces apoptosis in human pancreatic cancer cells. Anticancer Drugs. 2020;31:431–439. doi: 10.1097/CAD.0000000000000914. [DOI] [PubMed] [Google Scholar]
- 70.Rushworth LK, Hewit K, Munnings-Tomes S, Somani S, James D, Shanks E, Dufès C, Straube A, Patel R, Leung HY. Repurposing screen identifies mebendazole as a clinical candidate to synergise with docetaxel for prostate cancer treatment. Br J Cancer. 2020;122:517–527. doi: 10.1038/s41416-019-0681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen Q, Li Y, Zhou X, Li R. Oxibendazole inhibits prostate cancer cell growth. Oncol Lett. 2018;15:2218–2226. doi: 10.3892/ol.2017.7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang QL, Lian DD, Zhu MJ, Li XM, Lee JK, Yoon TJ, Lee JH, Jiang RH, Kim CD. Antitumor effect of albendazole on cutaneous squamous cell carcinoma (SCC) cells. BioMed Res Int. 2019;2019:3689517. doi: 10.1155/2019/3689517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Čáňová K, Rozkydalová L, Vokurková D, Rudolf E. Flubendazole induces mitotic catastrophe and apoptosis in melanoma cells. Toxicol In Vitro. 2018;46:313–322. doi: 10.1016/j.tiv.2017.10.025. [DOI] [PubMed] [Google Scholar]
- 74.Doudican NA, Byron SA, Pollock PM, Orlow SJ. XIAP downregulation accompanies mebendazole growth inhibition in melanoma xenografts. Anticancer Drugs. 2013;24:181–188. doi: 10.1097/CAD.0b013e32835a43f1. [DOI] [PubMed] [Google Scholar]
- 75.Simbulan-Rosenthal CM, Dakshanamurthy S, Gaur A, Chen YS, Fang HB, Abdussamad M, Zhou H, Zapas J, Calvert V, Petricoin EF, et al. The repurposed anthelmintic mebendazole in combination with trametinib suppresses refractory NRASQ61K melanoma. Oncotarget. 2017;8:12576–12595. doi: 10.18632/oncotarget.14990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li Y, Acharya G, Elahy M, Xin H, Khachigian LM. The anthelmintic flubendazole blocks human melanoma growth and metastasis and suppresses programmed cell death protein-1 and myeloid-derived suppressor cell accumulation. Cancer Lett. 2019;459:268–276. doi: 10.1016/j.canlet.2019.05.026. [DOI] [PubMed] [Google Scholar]
- 77.Doudican N, Rodriguez A, Osman I, Orlow SJ. Mebendazole induces apoptosis via Bcl-2 inactivation in chemoresistant melanoma cells. Mol Cancer Res. 2008;6:1308–1315. doi: 10.1158/1541-7786.MCR-07-2159. [DOI] [PubMed] [Google Scholar]
- 78.Williamson T, Mendes TB, Joe N, Cerutti JM, Riggins GJ. Mebendazole inhibits tumor growth and prevents lung metastasis in models of advanced thyroid cancer. Endocr Relat Cancer. 2020;27:123–136. doi: 10.1530/ERC-19-0341. [DOI] [PubMed] [Google Scholar]
- 79.Martarelli D, Pompei P, Baldi C, Mazzoni G. Mebendazole inhibits growth of human adrenocortical carcinoma cell lines implanted in nude mice. Cancer Chemother Pharmacol. 2008;61:809–817. doi: 10.1007/s00280-007-0538-0. [DOI] [PubMed] [Google Scholar]
- 80.Sawanyawisuth K, Williamson T, Wongkham S, Riggins GJ. Effect of the antiparasitic drug mebendazole on cholangiocarcinoma growth. Southeast Asian J Trop Med Public Health. 2014;45:1264–1270. [PubMed] [Google Scholar]
- 81.Sung SJ, Kim HK, Hong YK, Joe YA. Autophagy is a potential target for enhancing the anti-angiogenic effect of mebendazole in endothelial cells. Biomol Ther (Seoul) 2019;27:117–125. doi: 10.4062/biomolther.2018.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hassanpour M, Rezabakhsh A, Pezeshkian M, Rahbarghazi R, Nouri M. Distinct role of autophagy on angiogenesis: highlights on the effect of autophagy in endothelial lineage and progenitor cells. Stem Cell Res Ther. 2018;9:305. doi: 10.1186/s13287-018-1060-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kardideh B, Samimi Z, Norooznezhad F, Kiani S, Mansouri K. Autophagy, cancer and angiogenesis: where is the link? Cell Biosci. 2019;9:65. doi: 10.1186/s13578-019-0327-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bhutia SK, Mukhopadhyay S, Sinha N, Das DN, Panda PK, Patra SK, Maiti TK, Mandal M, Dent P, Wang XY, et al. Autophagy: cancer's friend or foe? Adv Cancer Res. 2013;118:61–95. doi: 10.1016/B978-0-12-407173-5.00003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mandhair HK, Arambasic M, Novak U, Radpour R. Molecular modulation of autophagy: New venture to target resistant cancer stem cells. World J Stem Cells. 2020;12:303–322. doi: 10.4252/wjsc.v12.i5.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Görgülü K, Diakopoulos KN, Kaya-Aksoy E, Ciecielski KJ, Ai J, Lesina M, Algül H. The role of autophagy in pancreatic cancer: from bench to the dark bedside. Cells. 2020;9:1063. doi: 10.3390/cells9041063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tan Z, Chen L, Zhang S. Comprehensive modeling and discovery of mebendazole as a novel TRAF2- and NCK-interacting kinase inhibitor. Sci Rep. 2016;6:33534. doi: 10.1038/srep33534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shitashige M, Satow R, Jigami T, Aoki K, Honda K, Shibata T, Ono M, Hirohashi S, Yamada T. Traf2- and Nck-interacting kinase is essential for Wnt signaling and colorectal cancer growth. Cancer Res. 2010;70:5024–5033. doi: 10.1158/0008-5472.CAN-10-0306. [DOI] [PubMed] [Google Scholar]
- 89.Walker JC, Harland RM. microRNA-24a is required to repress apoptosis in the developing neural retina. Genes Dev. 2009;23:1046–1051. doi: 10.1101/gad.1777709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sun Y, Yu X, Bai Q. miR-204 inhibits invasion and epithelial-mesenchymal transition by targeting FOXM1 in esophageal cancer. Int J Clin Exp Pathol. 2015;8:12775–12783. [PMC free article] [PubMed] [Google Scholar]
- 91.Rubin J, Mansoori S, Blom K, Berglund M, Lenhammar L, Andersson C, Loskog A, Fryknäs M, Nygren P, Larsson R. Mebendazole stimulates CD14+ myeloid cells to enhance T-cell activation and tumour cell killing. Oncotarget. 2018;9:30805–30813. doi: 10.18632/oncotarget.25713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bai RY, Staedtke V, Wanjiku T, Rudek MA, Joshi A, Gallia GL, Riggins GJ. Brain penetration and efficacy of different mebendazole polymorphs in a mouse brain tumor model. Clin Cancer Res. 2015;21:3462–3470. doi: 10.1158/1078-0432.CCR-14-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bai RY, Staedtke V, Rudin CM, Bunz F, Riggins GJ. Effective treatment of diverse medulloblastoma models with mebendazole and its impact on tumor angiogenesis. Neuro-oncol. 2015;17:545–554. doi: 10.1093/neuonc/nou234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Choi EK, Kim SW, Nam EJ, Paek J, Yim GW, Kang MH, Kim YT. Differential effect of intraperitoneal albendazole and paclitaxel on ascites formation and expression of vascular endothelial growth factor in ovarian cancer cell-bearing athymic nude mice. Reprod Sci. 2011;18:763–771. doi: 10.1177/1933719111398142. [DOI] [PubMed] [Google Scholar]
- 95.Pourgholami MH, Szwajcer M, Chin M, Liauw W, Seef J, Galettis P, Morris DL, Links M. Phase I clinical trial to determine maximum tolerated dose of oral albendazole in patients with advanced cancer. Cancer Chemother Pharmacol. 2010;65:597–605. doi: 10.1007/s00280-009-1157-8. [DOI] [PubMed] [Google Scholar]
- 96.Nygren P, Larsson R. Drug repositioning from bench to bedside: tumour remission by the antihelmintic drug mebendazole in refractory metastatic colon cancer. Acta Oncol. 2014;53:427–428. doi: 10.3109/0284186X.2013.844359. [DOI] [PubMed] [Google Scholar]
- 97.Dobrosotskaya IY, Hammer GD, Schteingart DE, Maturen KE, Worden FP. Mebendazole monotherapy and long-term disease control in metastatic adrenocortical carcinoma. Endocr Pract. 2011;17:e59–e62. doi: 10.4158/EP10390.CR. [DOI] [PubMed] [Google Scholar]
- 98.Morris DL, Jourdan JL, Pourgholami MH. Pilot study of albendazole in patients with advanced malignancy. Effect on serum tumor markers/high incidence of neutropenia. Oncology. 2001;61:42–46. doi: 10.1159/000055351. [DOI] [PubMed] [Google Scholar]
- 99.Malik K, Dua A. StatPearls. Treasure Island, FL: StatPearls Publishing StatPearls Publishing LLC.; 2020. Albendazole. [Google Scholar]
- 100.LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2012. Albendazole. [PubMed] [Google Scholar]
- 101.Hong ST. Albendazole and praziquantel: review and safety monitoring in Korea. Infect Chemother. 2018;50:1–10. doi: 10.3947/ic.2018.50.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pawluk SA, Roels CA, Wilby KJ, Ensom MH. A review of pharmacokinetic drug-drug interactions with the anthelmintic medications albendazole and mebendazole. Clin Pharmacokinet. 2015;54:371–383. doi: 10.1007/s40262-015-0243-9. [DOI] [PubMed] [Google Scholar]
- 103.LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2012. Mebendazole. [PubMed] [Google Scholar]
- 104.Pantziarka P, Bouche G, Meheus L, Sukhatme V, Sukhatme VP. Repurposing drugs in oncology (ReDO)-mebendazole as an anti-cancer agent. Ecancermedicalscience. 2014;8:443. doi: 10.3332/ecancer.2014.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Capece BP, Virkel GL, Lanusse CE. Enantiomeric behaviour of albendazole and fenbendazole sulfoxides in domestic animals: pharmacological implications. Vet J. 2009;181:241–250. doi: 10.1016/j.tvjl.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 106.Villar D, Cray C, Zaias J, Altman NH. Biologic effects of fenbendazole in rats and mice: a review. J Am Assoc Lab Anim Sci. 2007;46:8–15. [PubMed] [Google Scholar]
- 107.Gonzalez AE, Codd EE, Horton J, Garcia HH, Gilman RH. Oxfendazole: a promising agent for the treatment and control of helminth infections in humans. Expert Rev Anti Infect Ther. 2019;17:51–56. doi: 10.1080/14787210.2018.1555241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Čáňová K, Rozkydalová L, Rudolf E. Anthelmintic flubendazole and its potential use in anticancer therapy. Acta Med (Hradec Kralove) 2017;60:5–11. doi: 10.14712/18059694.2017.44. [DOI] [PubMed] [Google Scholar]
- 109.Theodorides VJ, DiCuollo CJ, Nawalinski T, Miller CR, Murphy JR, Freeman JF, Killeen JC, Jr, Rapp WR. Toxicologic and teratologic studies of oxibendazole in ruminants and laboratory animals. Am J Vet Res. 1977;38:809–814. [PubMed] [Google Scholar]
- 110.Al-Hadiya BM. Parbendazole. Profiles Drug Subst Excip Relat Methodol. 2010;35:263–284. doi: 10.1016/S1871-5125(10)35005-9. [DOI] [PubMed] [Google Scholar]
- 111.Sánchez S, Alvarez L, Sallovitz J, Lanusse C. Enhanced plasma and target tissue availabilities of albendazole and albendazole sulphoxide in fasted calves: evaluation of different fasting intervals. J Vet Pharmacol Ther. 2000;23:193–201. doi: 10.1046/j.1365-2885.2000.00265.x. [DOI] [PubMed] [Google Scholar]
- 112.Movahedi F, Li L, Gu W, Xu ZP. Nanoformulations of albendazole as effective anticancer and antiparasite agents. Nanomedicine (Lond) 2017;12:2555–2574. doi: 10.2217/nnm-2017-0102. [DOI] [PubMed] [Google Scholar]
- 113.Morris DL, Smith PG. Albendazole in hydatid disease--hepatocellular toxicity. Trans R Soc Trop Med Hyg. 1987;81:343–344. doi: 10.1016/0035-9203(87)90259-8. [DOI] [PubMed] [Google Scholar]
- 114.Eckardt K, Kaltenhäuser J, Kilb C, Seiler A, Stahlmann R. Relative potency of albendazole and its sulfoxide metabolite in two in vitro tests for developmental toxicity: the rat whole embryo culture and the mouse embryonic stem cell test. Reprod Toxicol. 2012;34:378–384. doi: 10.1016/j.reprotox.2012.05.037. [DOI] [PubMed] [Google Scholar]
- 115.Navarro M, Canut L, Carretero A, Cristofol C, Pérez-Aparicio FJ, Arboix M, Ruberte J. Developmental toxicity in rat fetuses exposed to the benzimidazole netobimin. Reprod Toxicol. 1999;13:295–302. doi: 10.1016/s0890-6238(99)00013-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Antitumorigenicity of benzimidazole anthelmintics on cancer cell lines
Antitumorigenicity of benzimidazole anthelmintics in animal tumor models