Figure 1.
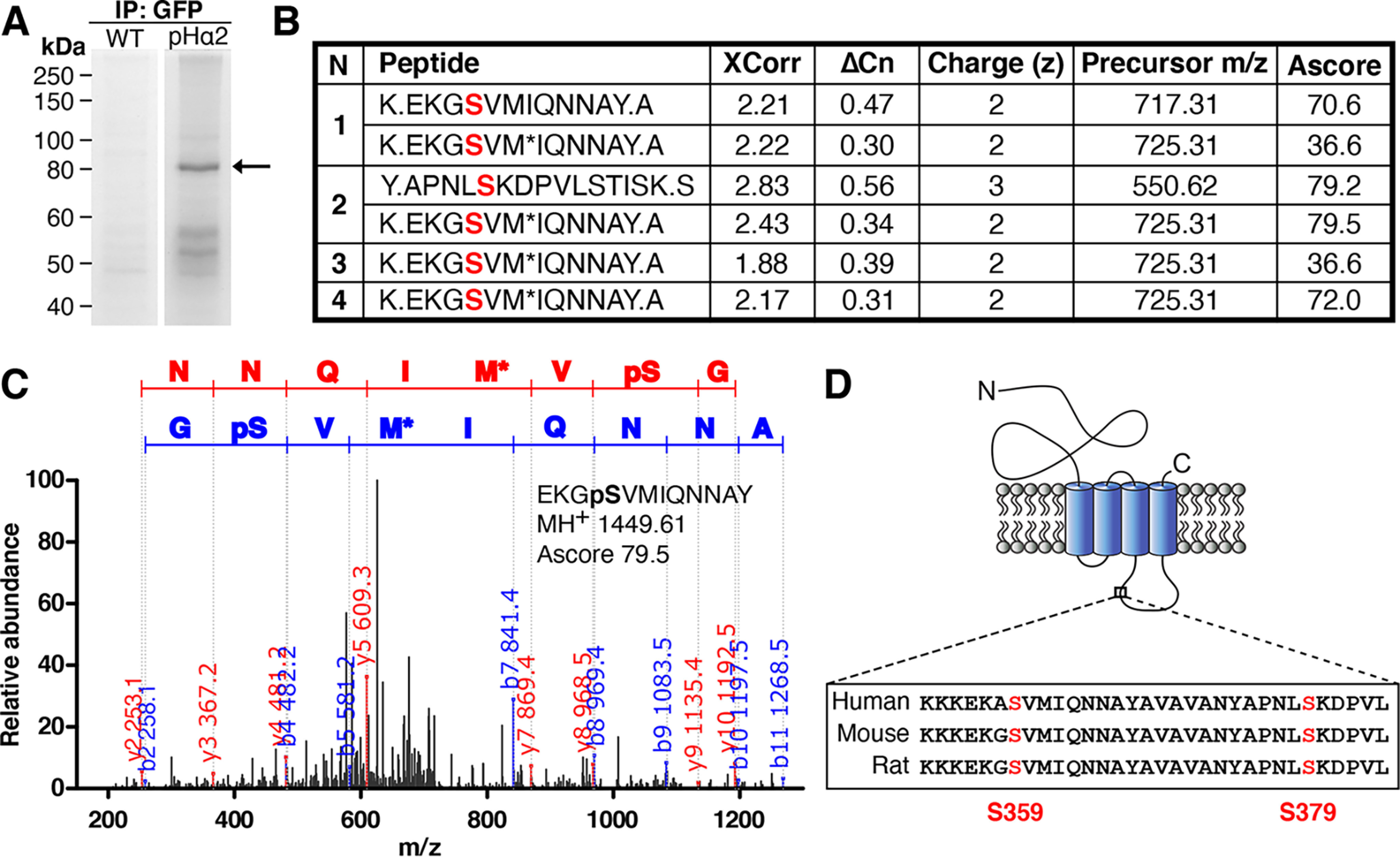
Identification of two novel phosphorylation sites on GABAAR α2 subunit at serine 359 and 379 by LC–MS/MS. A, neuronal lysates from WT and pHα2 mice were incubated with GFP-Trap and subjected to SDS-PAGE followed by Coomassie staining. The 80 kDa gel band (arrow) corresponding to pHα2 was excised and digested with both trypsin and chymotrypsin before MS analysis was performed by the Taplin MS Facility. B, GABAAR α2 phosphopeptide sequences identified by MS/MS analysis of pHα2, including the SEQUEST scores XCorr (cross-correlation) and ΔCn (delta correlation), charge state (z), precursor m/z, and Ascore values (for Ascore > 13, p < 0.05). Phosphorylated serine sites identified are colored red. M*, oxidation of methionine. A period in the peptide sequence indicates the protease cleavage sites. n = 4. C, MS/MS spectrum of the pHα2 derived for peptide EKGpSVMIQNNAY phosphorylated at Ser-359. The phosphorylated serine site is marked as pS. M*, oxidation of methionine. The observed b ion (blue) and y ion (red) peptide fragment masses are indicated on the spectrum. D, schematic depicting the GABAAR α2 subunit. The novel phosphorylated serine residues (red) are located between TM domains 3 and 4 on the large intracellular domain. Both identified serine sites are conserved in mice, rats, and humans.
