Figure 6.
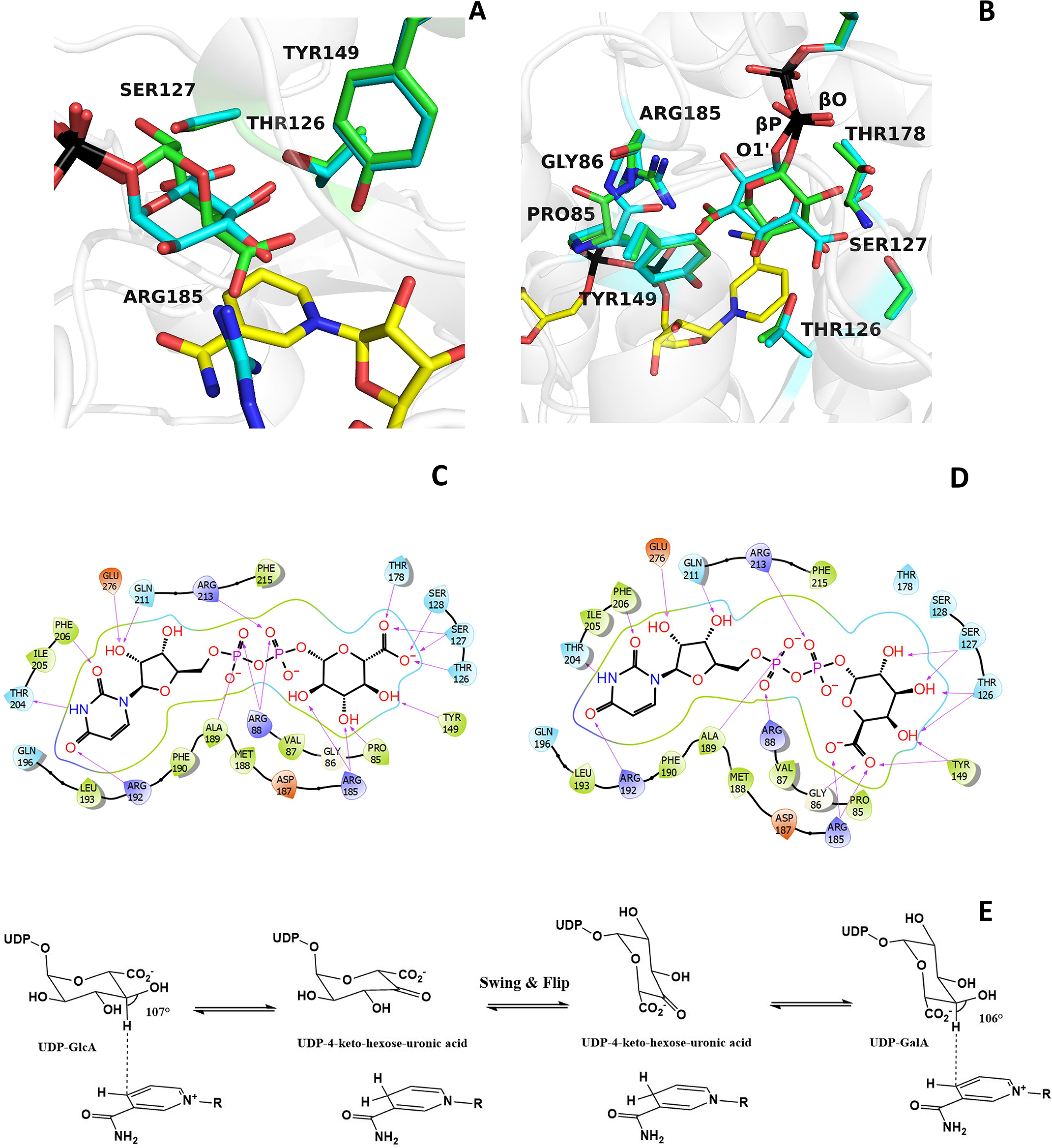
A detailed comparison of UDP-glucuronic and UDP-galacturonic acid binding. A and B, the galacturonic acid (green carbons) can be moved on top of the glucuronic acid (cyan carbons) through a rotation of about 160° around the bond between the β-phosphorus (βP) of UDP and O1′ of the sugar combined with a smaller “swinging” 30° rotation around the bond between the β-phosphorus and the pyrophosphate oxygen (βO) of UDP. These movements can be best-appreciated in the orientation of the right panel. The orientations are the same as in Fig. 3 (A and B). C and D, schematic two-dimensional overviews of the protein-ligand interactions. Despite their differing swing-rotated orientations, both sugars engage their polar groups in multiple hydrogen bonds with protein side chains. Only the 2′-carbon of glucuronic acid (B) is not hydrogen-bonded to the protein as it interacts with ordered waters. Moreover, the conformations of all active-site side chains remain virtually unaltered in the two complexes. E, scheme of the BcUGAepi reaction. The fine geometry of the C4′sugar–C4nicotinamide contact allows C4′ oxidation of glucuronate (forward reaction) and galacturonate (reverse reaction) acid.
