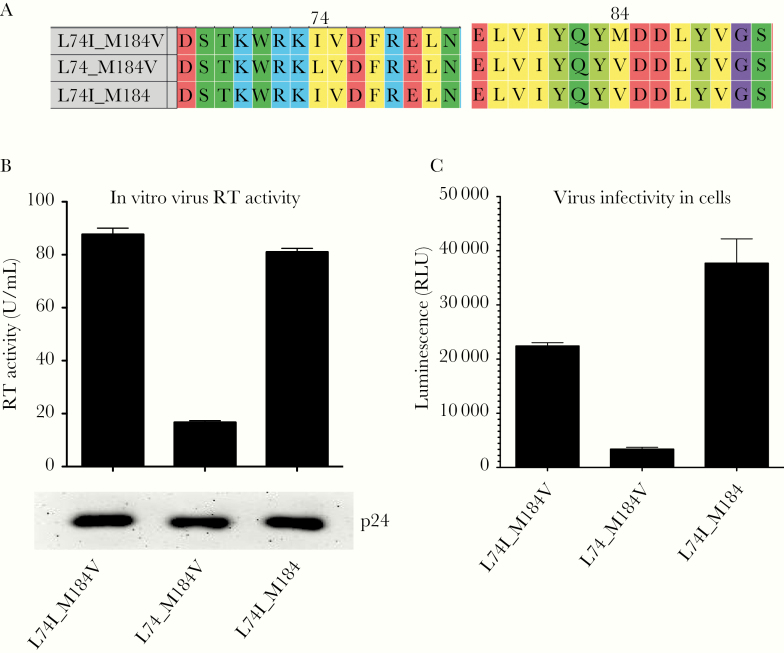
Figure 5.
In vitro replication measurement of lamivudine-resistant subtype C clinical isolate containing M184V and L74I and revertant mutations. (A) Amino acid multiple sequence alignment of clinical isolate and revertant mutants generated by site-directed mutagenesis. Numbering is relative to strain HXB2. (B) In vitro reverse-transcription (RT) efficiency contained in pelleted single-round virus from cells producing clinical human immunodeficiency virus (HIV) isolate RT sequence and mutants. Bottom panel shows Western blot of corresponding virus-associated p24 in supernatants from cells. (C) Single-round infection of target HEK 293T cells by equal quantities of luciferase expressing vesicular stomatitis virus-G pseudotyped HIV viruses from B. Data in B and C were performed in replicate, and means are presented with error bars corresponding to standard deviation. RLU, relative light units.