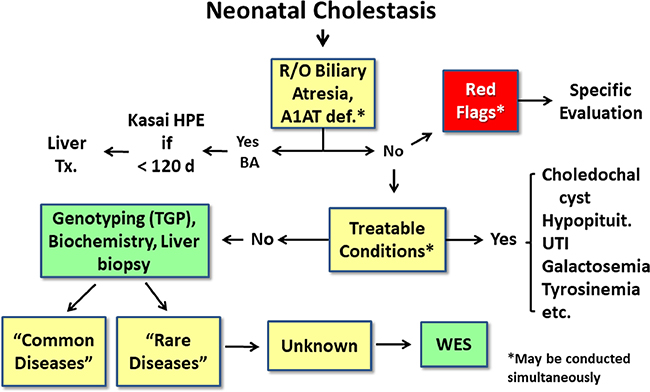
Figure 2.
Current paradigm for evaluation of neonatal cholestasis.In the current sequential evaluation of neonatal cholestasis, alpha-1 antitrypsin deficiency (A1AT), biliary atresia, and treatable causes of cholestasis (such as choledochal cyst or urinary tract infection) are excluded in a timely fashion, which may require liver biopsy and intraoperative cholangiogram to determine if biliary atresia is present. If a “red flag” points to a specific diagnosis (Table 2), evaluation for that disease should proceed promptly.If no diagnosis if found, the next tier of testing may include multiple blood and urine tests to evaluate for infectious, genetic and metabolic causes. If no diagnosis is confirmed, targeted gene panels or specific gene testing is performed and whole exome sequencing (WES) is reserved for those cases without a diagnosis after this exhaustive evaluation. R/O= rule out, HPE= hepatoportoenterostomy, UTI= urinary tract infection, PFIC= progressive familial intrahepatic cholestasis, TGP= targeted gene panel, WES= whole exome sequencing.