Figure 1. Mutations in the IN1 bNLS linker sequence abolish the interaction with AC40.
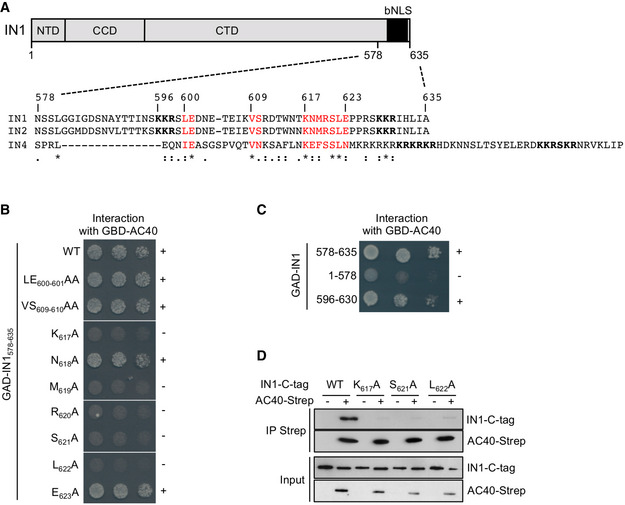
- Top. The Ty1 integrase (IN1) showing N‐terminal and catalytic core domains (NTD and CCD) and the bipartite NLS at the C‐terminus (CTD). Bottom. Alignment of amino acid sequences of Ty1, Ty2, and Ty4 integrase C‐termini (IN1, IN2, and IN4, respectively). In bold: Basic amino acids required for NLS function. In red: Amino acids in the bNLS linker relatively conserved between the three integrases. *, identity; :, high similarity; ., low similarity; ‐, gap in sequence.
- Two‐hybrid interaction between GBD‐AC40 and WT or mutant GAD‐IN1578–635. Alanine substitutions in IN1578‐635 are indicated. Cells were plated in twofold serial dilutions on DO‐Leu‐Trp‐His plates. No growth or protein expression defects were detected (Fig EV1A and B). +, interaction; −, no interaction.
- Two‐hybrid interaction between GBD‐AC40 and different IN1 regions fused to GAD, as indicated. Cells were plated in ten‐fold serial dilutions on DO‐Leu‐Trp‐His plates. No growth or protein expression defects were detected (Fig EV1C and D). +, interaction; −, no interaction.
- In vitro interaction between AC40 and IN1 proteins co‐expressed in E. coli. Co‐precipitation of WT or mutated (K617A, S621A, or L622A) IN1‐C‐tag using AC40‐Twin‐Strep‐tag as bait from bacterial protein extracts co‐expressing (+) or not (−) these proteins. Expected sizes are 41 kDa for AC40‐Twin‐Strep‐tag and 100 kDa for IN1‐C‐tag (WT and mutants).
Source data are available online for this figure.
