FIG 1.
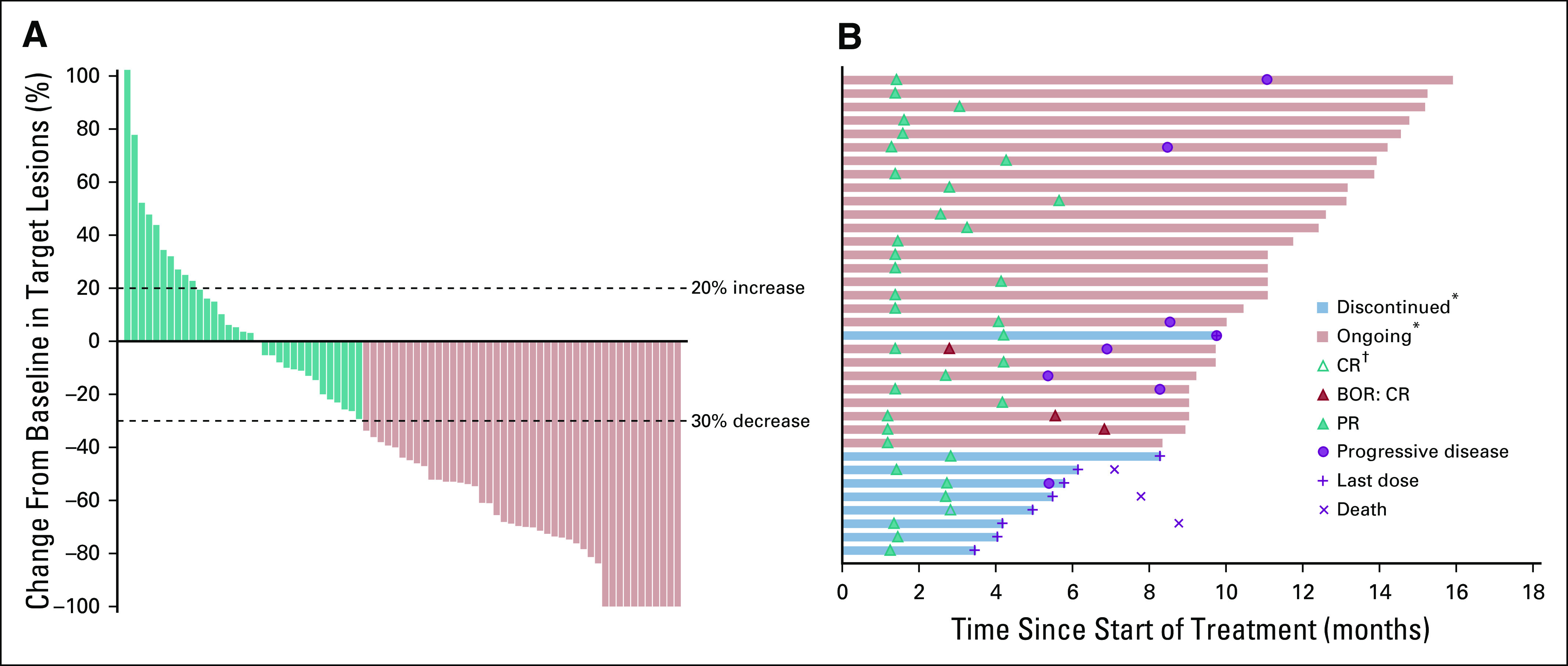
(A) Best percentage change from baseline in target lesion and (B) duration of study treatment and response in responders (n = 36) in all patients as treated. All patients had at least one postbaseline assessment of target lesion(s) (n = 76). Symbols for complete response (CR), partial response (PR), and progressive disease depict the first response to pembrolizumab. Symbols depict the timing of first objective response unless otherwise indicated. (*) Discontinued or ongoing refers to status in relation to study treatment. (†) Patient achieved a best overall response (BOR) of CR.
