Abstract
The unprecedented use of antibiotics that led to development of resistance affect human health worldwide. Prescription of antibiotics imprudently and irrationally in different diseases progressed with the acquisition and as such development of antibiotic resistant microbes that led to the resurgence of pathogenic strains harboring enhanced armors against existing therapeutics. Compromised the treatment regime of a broad range of antibiotics, rise in resistance has threatened human health and increased the treatment cost of diseases. Diverse on metabolic, genetic and physiological fronts, rapid progression of resistant microbes and the lack of a strategic management plan have led researchers to consider plant-derived substances (PDS) as alternative or in complementing antibiotics against the diseases. Considering the quantitative characteristics of plant constituents that attribute health beneficial effects, analytical procedures for their isolation, characterization and phytochemical testing for elucidating ethnopharmacological effects has being worked out for employment in the treatment of different diseases. With an immense potential to combat bacterial infections, PDSs such as polyphenols, alkaloids and tannins, present a great potential for use, either as antimicrobials or as antibiotic resistance modifiers. The present study focuses on the mechanisms by which PDSs help overcome the surge in resistance, approaches for screening different phytochemicals, methods employed in the identification of bioactive components and their testing and strategies that could be adopted for counteracting the lethal consequences of multidrug resistance.
Keywords: antibiotics, bacteria, human health, plant-derived substances, resistance
1. Introduction
Antibiotics are natural or synthetic organic molecules that are effective against microorganisms. With propensity to human use as defense armor, they provide protection against infections caused by bacteria and fungi. The emergence of resistance among microbes has limited the effectiveness of antibiotics, shifting the lifesaving paradigm built around them. This prominent trend is regarded as a matter of concern by agencies like the World Health Organization and has become a challenging situation for the medical fraternity worldwide [1]. Despite incredible advancement in the scientific and modern medicine, the hopefulness of antibiotics is faded away by the appearance of different resistance mechanisms that presents a grave concern against the frontline antibiotics. Of them, antibiotic inactivation via, production of a range of enzymes, change in cell permeability, alteration of drug targets, intrinsic expression of efflux pumps and biofilm formation that acts as defense against drugs and contribute to sustained persistence of resistance bacteria [2,3,4,5,6,7]. In addition to mutations, mobile genetic elements (plasmids, insertion sequences, transposons and integrative conjugative elements) play a crucial role in the expansion of resistance among diverse groups of bacteria [8]. Occurrence of resistance genes in bacteria favors their propagation and perpetuation in new territories. Contributing a unique niche for the growth of microorganisms, their establishment in the oral cavity especially dental surfaces serves as etiological agents of dental caries [9,10,11]. There were a large number of reports that suggest irrational enhancement in prescribing antibiotics by medical professionals including dental surgeons that in turn contributes significantly amount to the growing burden of microbial resistance [12,13,14]. Confronted by increasing amounts of antibiotics use, appearance of opportunistic organisms intrinsically resistant to drugs that thwarts currently available treatment regimes makes diseases difficult and, sometimes, impossible to treat in hospitals and the community settings.
An extensive increase in antibiotic resistance owed to a sustained persistence of resistant bacteria is becoming a serious threat for public health worldwide. The situation is aggravated by the decline in the production of drugs since the late 1960 s and by the long periods of time needed for testing new drugs before acceptance by the authorities for commercialization [15]. Under these circumstances, there is a growing need for identifying alternatives to antibiotics in the prevention and treatment of microbial infections. One such approach is the utilization of naturally occurring botanicals that have the potential to be used as an alternative or a complement to antibiotics. Conventional curative systems have counted upon traditional herbs that are rich in compounds, such as alkaloids, terpenoids, tannins, steroids, coumarins and flavonoids [16], and do not normally cause resistance [17]. Essential oils (Eos) from parsley, lovage, basil and thyme disrupt the physiological status of the bacterial cell by causing an increase in cell permeability, leakage of cell constituents, alterations in bacterial cell wall and cell membrane, ATP loss, inhibition of protein synthesis, pH disturbance, intracytoplasmic damage, DNA damage and inhibition of quorum sensing among bacteria like Bacillus cereus, Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia. coli and Salmonella enterica serovar Typhimurium [18,19]. The present review summarizes the approaches for screening different phytochemicals, methods employed in the identification of bioactive components and their testing, strategies adopted along with the mechanistic insights of plant-derived substances (PDSs) in counteracting the lethal consequences of multidrug resistance.
2. Isolation and Characterization of Bioactive Compounds
Different approaches are employed for screening the bioactive constituents of PDSs. The direct method involves total chemical characterization of a plant species using approaches of dereplication (identification of already known bioactive constituents) via liquid chromatography–mass spectrometry (LC–MS). The objective is to isolate novel plant compounds, which are then sent directly for biologic testing [20,21,22,23,24]. Isolated plant compounds are collected for libraries, such as the National Cancer Institute’s Natural Product Repository [25]. Libraries can be screened to test the bioactivities of various compounds in order to examine fully characterized, new natural products [26,27,28]. Bioactivity-guided fractionation method uses bioassays as fractionation monitors; uninterrupted fractionation cycles are used together for testing bioactive extracts so that pure and active principle compounds are isolated. This is the most commonly used way of determining bioactives. Despite significant increase in the number of studies reporting bioactives [29,30,31,32] through this process, a large number of botanicals and their preparations are still in use without any knowledge of their bioactives [33,34,35,36]. The synergy directed fractionation method uses bioactivity-guided fractionation combined with bioactivity testing in order to understand synergistic connections between the compounds present in a mixture. The method uses mass spectrometry (MS)-profiling for a guided isolation of natural products, targeting the synergistic interactions among obtained extracts which could not have been taken into consideration through conventional guided fractionation [37,38,39,40,41]. Metabolism directed method is focused on the identification of bioactive metabolites not present at the beginning of the test, but produced with time due to changes in the metabolism of the plant or plant organ under study [42,43,44,45,46,47,48,49]. Additionally, metabolic profiling (metabolomics) method correlates chemical profiling of plant extracts with isolation and identification of new or already known bioactive constituents at an early stage (dereplication) [50,51]; the goal of metabolomics in general is to analyze all secondary metabolites in a sample, both qualitatively and quantitatively. Comparison of bioactivity data permits early stage dereplication. This method also gives information about possible synergistic effects between molecules [52,53,54,55,56,57,58].
3. Screening of PDSs for Drug Discovery
Plant materials are selected on the basis of habitual interactions between plants and the environment, taking into consideration that synthesized secondary metabolites may possess significant therapeutic benefits for humans. The screening of PDSs for drug discovery includes random, ethnopharmacological and computational approaches. Random selection of extracts is based on accessibility of plants and their enriched fractions. It unravels unexpected bioactivities that could have been missed based on the present knowledge [59,60,61,62,63]. As pharmacological assays have medium to small throughput and the starting test samples (extracts, fractions or pure compounds) are available only in small amounts, the number of bioassays through which they can be tested is limited. The ethnopharmacological approach relies mainly on the traditional medicinal applications of plant species [34,64,65,66,67]. This approach uses the observation, description and experimental investigation of traditionally used drugs and their bioactivities. It involves an interdisciplinary approach, including botany, biochemistry, chemistry, pharmacology and other fields beyond natural sciences like anthropology, archeology, history and linguistics [68,69,70]. In computational approach, plant materials or natural products are selected based on the prediction of their bioactive constituents with a high likelihood for biologic activity. In addition, to predict characteristics of molecular structures like protein–ligand biding interactions, in silico simulations can be used. Various computational approaches have been reported, such as the QSAR model (quantitative structure activity relationship); [71,72], which is used to predict compounds with high specificity for targets. Bioactivity databases, such as CHEMBL [73] or PubChem [74] are used to evaluate datasets for compatibility among naturally obtained products and synthetically obtained pharmacologically active molecules. The pharmacophore model [75,76] simulates 3D arrangements of molecules with different physicochemical features involved in the interactions between a ligand and its target. Meanwhile, molecular docking has a crucial function in defining drug–protein interactions, helpful for determining whether plant extracts/compounds act as substrates for efflux pump proteins. Molecular docking suggests structure–activity relationships of natural products for elucidating their mechanism of action and predicting the positioning of a ligand within a protein-binding pocket [77].
4. Bioassays for Phytochemical Testing
Gas chromatography–mass spectrometry (GC–MS) techniques are commonly used for the analysis of phytochemicals in plant extracts [78,79,80,81,82]. Due to its high selectivity and sensitivity in the analysis of chemical constituents of plant origin, GC–MS is considered as the gold standard for the elucidation of phytochemical profiles of plant extracts. In addition, agar macrodilution and microdilution methods are generally used to determine the minimum inhibitory concentration (MIC) of plant extracts against different bacterial isolates (Table 1).
Table 1.
Plant-derived substances and their action against different microbes.
| Plant Name | Plant Derivatives | Bacterial sp. | * MIC Value | References |
|---|---|---|---|---|
| Anogeissusa cuminata | terpenoids, flavonoids, saponins, tannins, alkaloids | S. aureus | 0.29 mg/mL | [83] |
| A. baumannii | 1.51 mg/mL | |||
| C. freundii | 3.41 mg/mL | |||
| E. coli | 3.41 mg/mL | |||
| K. oxytoca | 1.51 mg/mL | |||
| K. pneumoniae | 0.67 mg/mL | |||
| P. aeruginosa | 0.67 mg/mL | |||
| Azadirachta indica | β-sitosterol, flavonoids | S. aureus | 3.41 mg/mL | [83] [84] |
| A. baumannii | 4.27 mg/mL | |||
| C. freundii | 3.41 mg/mL | |||
| E. coli | 4.27 mg/mL | |||
| K. oxytoca | 9.63 mg/mL | |||
| K. pneumoniae | 4.27 mg/mL | |||
|
P. aeruginosa
H. pylori |
9.63 mg/mL 128 µg/mL |
|||
| Bauhinia variegata | terpenoids, flavonoids, tannins, saponins, glucoside, | S. aureus | 3.41 mg/mL | [83] |
| A. baumannii | 9.63 mg/mL | |||
| C. freundii | 9.63 mg/mL | |||
| E. coli | 4.27 mg/mL | |||
| K. oxytoca | 9.63 mg/mL | |||
| K. pneumoniae | 4.27 mg/mL | |||
| P. aeruginosa | 3.41 mg/mL | |||
| Boerhaavia diffusa | β-sitosterol, flavonoids | S. aureus | 4.27 mg/mL | [83] |
| A. baumannii | 9.63 mg/mL | |||
| C. freundii | 9.63 mg/mL | |||
| E. coli | NA | |||
| K. oxytoca | 9.63 mg/mL | |||
| K. pneumoniae | 9.63 mg/mL | |||
| P. aeruginosa | 9.63 mg/mL | |||
| Punica granatum | flavonoids, ellagitannin, punicalagin, ellagic acid | S. aureus | 0.29 mg/mL | [83] |
| A. baumannii | 3.41 mg/mL | |||
| C. freundii | 0.67 mg/mL | |||
| E. coli | 0.67 mg/mL | |||
| K. oxytoca | 3.41 mg/mL | |||
| K. pneumoniae | 3.41 mg/mL | |||
| P. aeruginosa | 0.67 mg/mL | |||
| Soymida febrifuga | methyl angolensate, luteolin 7-O-glucoside, flavonoid, sitosterol, myricetin | S. aureus | 067 mg/mL | [83] |
| A. baumannii | 1.51 mg/mL | |||
| C. freundii | 3.41 mg/mL | |||
| E. coli | 4.27 mg/mL | |||
| K. oxytoca | 3.41 mg/mL | |||
| K. pneumoniae | 3.41 mg/mL | |||
| P. aeruginosa | 4.27 mg/mL | |||
| Terminalia chebula | flavonoids and flavins, terpenoids, steroids, alkaloids, tannins and their derivatives, glycosides | S. aureus | 1.51 mg/mL | [83] |
| A. baumannii | 9.63 mg/mL | |||
| C. freundii | NA | |||
| E. coli | 9.63 mg/mL | |||
| K. oxytoca | 9.63 mg/mL | |||
| K. pneumoniae | 9.63 mg/mL | |||
| P. aeruginosa | 9.63 mg/mL | |||
| Tinospora cordifolia | alkaloids terpenoids, lactones, glycosides, steroids, phenolics | S. aureus | 4.27 mg/mL | [83] |
| A. baumannii | NA | |||
| C. freundii | 9.63 mg/mL | |||
| E. coli | 4.27 mg/mL | |||
| K. oxytoca | 9.63 mg/mL | |||
| K. pneumoniae | 9.63 mg/mL | |||
| P. aeruginosa | 4.27 mg/mL | |||
| Tribulus terrestris | flavonoids, flavonol glycosides, steroidal saponins and alkaloids | S. aureus | 3.41 mg/mL | [83] |
| A. baumannii | 9.63 mg/mL | |||
| C. freundii | 9.63 mg/mL | |||
| E. coli | 4.27 mg/mL | |||
| K. oxytoca | 4.27 mg/mL | |||
| K. pneumoniae | 9.63 mg/mL | |||
| P. aeruginosa | 3.41 mg/mL | |||
| Petroselinum crispum EO (Essential oil) | phenolic, flavonoids, coumarins | B. cereus | 22.68 µL/mL | [85] |
| S. aureus | 10.80 µL/mL | |||
| P. aeruginosa | 47.62 µL/mL | |||
| E. coli | 10.80 µL/mL | |||
| S. typhimurium | 47.62 µL/mL | |||
| Levisticum officinale EO | terpenoids, n-butylidene phthalide n-butyl-phthalide, sedanonic anhydride, d-terpineol, l, l phenolic, | B. cereus | 47.62 µL/mL | [85] |
| S. aureus | 2.45 µL/mL | |||
| P. aeruginosa | 22.68 µL/mL | |||
| E. coli | 10.80 µL/mL | |||
| S. typhimurium | 47.62 µL/mL | |||
| Occimomum basilicum EO | rosmarinic acid, phenol and terpenoid | B. cereus | 10.80 µL/mL | [85] |
| S. aureus | 2.45 µL/mL | |||
| P. aeruginosa | 22.68 µL/mL | |||
| E. coli | 10.80 µL/mL | |||
| S. typhimurium | 22.68 µL/mL | |||
| Thymus vulgare EO | p-cymene, γ-terpinene, thymol | B. cereus | 0.56 µL/mL | [85] |
| S. aureus | 0.06 µL/mL | |||
| P. aeruginosa | 0.56 µL/mL | |||
| E. coli | 0.27 µL/mL | |||
| S. typhimurium | 0.56 µL/mL | |||
| Cannabis sativa L., EO | phenol, flavonoid | S. aureus | 8 mg/mL | [86] |
| Acrosta phylosuvaursi | ellagic and gallic acid tannins, flavonoids, phenol | S. aureus | 90 µg/mL | [87] |
| Coptis chinensis | isoquinoline, alkaloids | 121 µg/mL | ||
| Eucalyptus globulus | 1,8-cineole, α-pinene, p-cymene | 118 µg/mL | ||
| Larreatri dentata | alkaloids, lignans, flavonoid, terpenoids | 60 µg/mL | ||
| Alpinia galanga | α-pinene, myrcene, limonene |
Mycobacterium smegmatis mc2 155 |
3.12–25 mg/L | [88] |
| Ammannia spp. | dioxyflavanol, quercetin and kaempferol | E. coli | 125 µg/mL | [89] |
| Berberis vulgaris | isoquinoline, alkaloids, | P. aeruginosa | 250–1000 µg/mL | [90] |
| Acer saccharum | flavonoids, tannins | E. coli | 5 and 10 mg/mL | [91] |
| P. aeruginosa | ||||
| P. mirabilis | ||||
| Catharanthus roseus | limonene, terpenoid | P. aeruginosa | 25 mg/L | [92] |
| Holarrhena antidysenterica | triterpenoids, sitosterol, phytosterol | P. aeruginosa | 20 mg/L | [93] |
| Cuminum cyminum | alkaloid, phenols, flavonoid, glycoside, saponin, tannin and steroid | S. aureus | 5 mg/mL | [94] |
| Salvia fruticosa | flavonoids, phenolics and rosemarinic acid | S. epidermidis | 5 µl/mL | [95] |
| Chenopodium Ambrosioides | α-terpinene, ascaridole | S. aureus | 170.6 µl/mL | [96] |
| Terminalia chebola | ellagic acid, gallic acid | E. coli | 12.1–97.5 µg/mL | [97] |
| Persea lingue | flavonoids, | S. aureus | 1.56 mg/L | [98] |
| Ipomoea muricata | ipomine, ipalbine, ipalbidine and ipalbinium | E. coli | 10 µg/mL | [99] |
| Hypericum olympicum | essential oils, α-pinene, β-ocimene, β-caryophyllene, germacrene-D | S. aureus | 50 µM | [100] |
| Alkanna orientalis | β-eudesmol, α-eudesmol and γ-eudesmol | S. aureus | 100 µM | [101] |
| Eucalyptus tereticornis | saponins, tannins, steroids flavonoids, cardiac glycosides | E. coli | 25 and 50 µg/mL |
[89] |
| Salvia officinalis | phenols, terpenoids |
E. cloacae, E. coli, S. typhimurium, P. aeruginosa, B. cereus |
0.01 mg/mL, 0.045 mg/mL, 0.045 mg/mL, 0.045 mg/mL, 0.09 mg/mL |
[102] |
* MIC corresponds to effect observed for the plant extract against given bacterial isolates.
Though screening of phytochemicals for antimicrobial activity is usually done by the agar well diffusion method, time-kill assay and lysis experience, commonly employed bioassay methods and new methodologies adopted in drug discovery includes in vitro assays (based on purified proteins, cell and target oriented), in situ assay (assay with isolated tissues and organs) and in vivo assays with model organisms.
4.1. In Vitro Assays
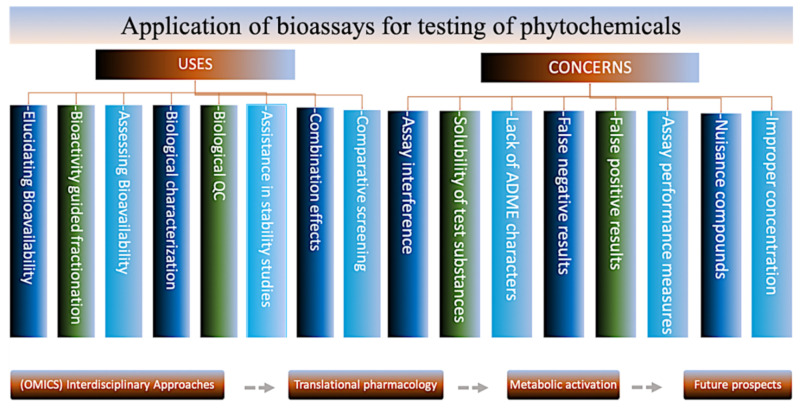
Assay with purified proteins relies on measuring either the pattern of interaction of a test compound with the target protein or the functional activity of the target protein in the presence of a test compound [64] (Figure 1). Allowing high throughput screening, it does not require cell culture or animal facilities. The method has a limitation of showing unrelated interaction between protein and the compound being tested. Cell-based assay is used to verify the activity of compounds at the cellular level. It may also unveil molecular mechanisms underlying certain biologic effects, leading to new target molecule discovery or pathways affecting the respective phenotype [64]. It provides medium to high throughput data and shows hits at the cellular level that are helpful in understanding the mechanisms of action involved and that can be used to discover novel molecular pathways to study concerned phenotypes. However, the method demands maintenance of the cell cultures and requires thorough knowledge and tedious efforts to reveal molecular targets affected in order to learn about the changed phenotypes and does not provide efficiency at in vivo level. Cell-based target oriented assay provokes the inhibition of a protein–protein interaction to check the functional activity or the activation of a protein upon binding to a compound [64]. The method provides knowledge of the molecular targets that helps in understanding the mechanism of action. It allows high throughput screening, demonstrating the efficiency of a target and giving information at a cellular level. The method has a prerequisite of cell culture and does not guarantee in vivo effectiveness of the compound being tested.
Figure 1.
Bioassays for testing Phytochemicals—Uses and concerns.
4.2. In Situ Assay with Isolated Tissues or Organs
The assay works at the interface of in vitro and in vivo models, encompassing an in situ method for isolated tissues or organs [103,104]. The method holds a high pathologic and physiological significance that reduces animal usage and allows high throughput in contrast to rodent models. However, the method provides low throughput in contrast to cell-based assays and have issues related to ethical clearance due to animal usage. Additionally, the isolated tissues and organs have a short ex vivo half-life.
4.3. In Vivo Assays Based on Model Organisms
Rodent models are employed for checking the in vivo efficacy of a drug, the discovery of new pharmacological targets and in the elucidation of the mechanisms of action of pharmacologically active compounds [105,106,107]. Having high homology with respect to humans, the method promises of the high pathophysiological significance of hits in a whole organism. Limitations of the method include slow throughput, requirement of ethical clearance and demand of having animal facility. Much time is needed to follow-up the work until the identification of the targets at a molecular level is achieved. Caenorhabditis elegans and the zebrafish (non-mammalian animal models) are next in the usage due to their widespread availability and the existence of instruments that support high throughput screening [108,109]. Their applicability as disease models is further expanded by the recent implementation of gene editing techniques [110]. These models are of high pathophysiological importance as they involve whole organisms. They could give rise to transgenic models. They require less amounts of the test substances in comparison to rodent models and are cost-effective. However, much time is needed to follow up on the work until the identification of the affected molecular target, besides detecting species-specific effects that are not relevant to humans. Galleria mellonella is used for the evaluation of pathogenesis and is used to study the potential of antimicrobial compounds. Galleria mellonella provides advantages of being used to test various bacterial species in a low time range with the cost-effectiveness manner. The management of this model comes easy. These qualities make Galleria mellonella an excellent model to check the in vivo efficacy of novel therapeutic remedies and simultaneously studying the host–pathogen interactions. Galleria mellonella is still at its infancy because the procedures for this model are not fully standardized [111].
5. Mechanistic Insights of Research on Botanicals
Plants synthesize a huge collection of structurally different compounds (polyphenols, terpenoids, essential oils, lectins, polypeptides and alkaloids), each having a specific and distinct role in the defense of the plant. Serving as potent contenders for future medicines, plant-based metabolites interfere with bacteria through different mechanisms. These are listed below.
5.1. Inhibition of Cell Wall Synthesis
Peptidoglycan is composed of repeating units of N-acetylmuramic acid (NAcMur) and N-acetylglucosamine (NAcGlc) residues cross-linked by short chains of amino acids. The sequence of amino acid residues plays a crucial role in terms of providing strength, and as such, protection to bacteria [112]. Plant-derived compounds have proved useful in the improvement of therapeutic methods to control the synthesis of the bacterial cell wall. The stamped antimicrobial property of flavonoids against an assortment of bacterial and contagious pathogens is attributed towards their action on the microbial cell wall [113]. Their interaction with membrane proteins attached with bacterial cell walls prompts expanded membrane penetrability and disturbance. Quinone, for example—anthraquinone from Cassia italica—was observed to be bacteriostatic against pathogenic microbes like Bacillus anthracis, Corynebacterium pseudodiphthericum and Pseudomonas aeruginosa and bactericidal towards Burkholderia pseudomallei. Cell wall lysis has likewise been recorded in bacteria presented to phenolic mixes. Flavones, flavonoids and flavonols, their action is most likely because of their capacity to complex with bacterial cell walls along with the extracellular and dissolvable proteins (Table 2).
Table 2.
Mechanism of action of plant-derived substances (PDSs) against broad range of microbes.
| Phytochemicals | Extract | Mode of Action | Antimicrobial Resistant Microbes | References |
|---|---|---|---|---|
| Flavonoids | Vaccinium macrocarpon Alt (cranberry) | Modifies biofilm formation | Enterococcus faecalis, E. coli, Pseudomonas aeruginosa | [114,115,116] |
| Myricetin, robinetin, epigallocatechin |
Blocks bacterial DNA synthesis | E. coli | [117] | |
| Quercetin | Inhibit ATPase activity, GrYB protein, elevates extracellular phosphatase and β-galactosidase | E. coli, S. aureus | [118] | |
| Plant-derived peptides | Moringa oleifera | Membrane disruption |
E. coli, S. aureus, P. aeruginosa, S. Typhimurium |
[119] |
| Essential oils (EOs) |
Petroselinum crispum EO, Levisticum officinale EO, Ocimum basilicum EO, Thymus vulgaris EO Cannabis sativa L., EO |
Increase cell permeability, leakage of cell constituents, alteration of bacterial cell wall and membrane disturbance, ATP loss, inhibit protein synthesis, lead to pH disturbance, intracytoplasmic damage, DNA damage, inhibit quorum sensing Inhibits biofilm formation |
Bacillus cereus, Staphylococcus aureus, P. aeruginosa, E. coli, S. Typhimurium S. aureus |
[120,121] [102] |
| Tea tree oil (TTO) | terpenes, monoterpenes, sesquiterpenes |
disrupts membrane permeability, damages cell membrane, obstructs cell growth, cause cell death |
E. coli, S. aureus, C. albicans |
[122,123] |
| Natural Efflux pump inhibitors (EPIs) | reserpine, gallotannin, piperine, curcumin, berberine, chalcones, carnosic acid |
Inhibit various efflux pump in bacteria (EtBr EP, MexAB-OprM) | MDR Uropathogenic E. coli, MDR, P. aeruginosa (clinical isolates) | [82,90,97,121,124,125] |
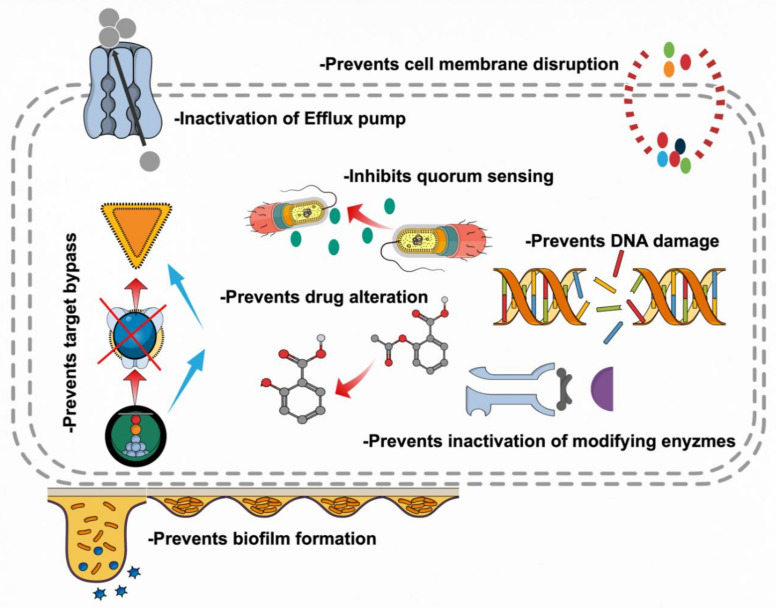
Increasing lipophilic flavonoids may likewise upset bacterial membranes. Investigation with scanning electron microscopy (SEM), subsequent to applying the flavonoid glycosides, showed that Pseudomonas aeruginosa cells began to distort at 30 min. At 60 min, the cells were totally twisted, in this manner recommending that the system of activity is through framing pores in the cell wall and harming the cell wall [126]. Tannins have properties that repress the development and protease action of ruminal microorganisms by targeting cell wall of bacteria. They could likewise disturb cell layers if lipophilic enough [127]. Alkaloids for the most part display antimicrobial action through intercalating into the cell wall and DNA of bacteria. At the point when galangin was joined with amoxicillin, transmission electron microscopy uncovered the detachment of the external membrane of the cells; a conceivable instrument is harm to the inside peptidoglycan layer. Amoxicillin and kaempferide or kaempferide-3-O–B-D-glucoside had an expanded hole between the external and cytoplasmic layers. These cells additionally exhibited morphologic harm to the cell wall and its shape. A few bacteria had broken cell walls. Amoxicillin or flavonoids alone were unfit to adjust the penetrability of the external layer as opposed to their blends. The impacts of consolidating amoxicillin and kaempferide or kempferide-3-O–B-D-glucoside were more powerful than amoxicillin and galangin [128,129]. Increment in porousness of membrane and disturbance is seen because of their association with bacterial cell wall as well as with membrane proteins present on it (Figure 2).
Figure 2.
Phytochemicals as antimicrobials—mode of action and their effectiveness against microbes.
5.2. Inhibition of Bacterial Physiology
Though the mechanisms of action of plant-derived substances (PDSs) is not clear, it is believed that they interfere with the organization of the cellular membrane leading to a diminished membrane potential and lower levels of ATP synthesis. Addition of PDSs to the medium causes changes in membrane permeability, metal ion chelation and disruption in the activity of membrane-bound ATPase that changes the physiological state of the bacteria and resulting in bacterial death [130,131,132,133,134,135]. It has been observed that thymol, carvacrol, catechins and eugenol disrupt the membrane structure and cause the discharge of cellular components, leading to depletion of cellular ATP [132,133,134,135]. Cinnamaldehyde also provokes a depletion of intracellular ATP by hampering ATPase-dependent metabolism, besides inhibiting glucose uptake and consumption [132,133,134,135]. Moreover, tea tree oil, composed of terpenes, monoterpenes, sesquiterpenes and the alcohols 1,8-cineol, α-terpineol and terpinen-4-ol, has the capacity to disrupt membrane permeability, damage cell membrane and obstruct cell growth, causing cell death in resistant microbes, like Escherichia coli, Staphylococcus aureus and Candida albicans [136].
5.3. Modulation of Antibiotic Susceptibility
Researchers has discovered that PDSs have a built-in antimicrobial potency and can be effective as resistance modulating compounds. Geraniol, an essential oil from Helichrysum italicum, can reinstate the efficiency of chloramphenicol, quinolones and β-lactams towards multidrug resistant bacteria Acinetobacter baumannii [137]. Comparable synergism was seen among antibiotics and several medicinal plant extracts, such as those from Camellia sinensis [138] and Cesalpinia spinosa [139], the oil of Croton zehntneri [140] and compounds like carvacrol [141] and baicalein derived from Scutellaria baicalensis [142].
The control mechanisms of plant compounds for bacteria are being studied due to their participation in reducing the effect of strategies used by bacteria to combat the effects of antibiotics (including enzymatic degradation and alteration of target sites and efflux pumps (EPs) [143]. Moreover, the combination of antibiotics and PDSs may be used in therapies to inhibit many pathways required for normal bacterial infection mechanisms and for reducing the synthesis of bacterial virulence factors. While extracts from Bridelia micrantha and Garcinia lucida restrict activity of β-lactamases [144], inhibition of the penicillinase activity by epigallocatechin gallate from green tea in methicillin-resistant Staphylococcus aureus (MRSA) restores antibacterial properties of both penicillin and ampicillin [145]. As several PDSs were found having an inhibitory effect on EPs, investigations on eugenol, β-resorcylic acid, trans-cinnamaldehyde, thymol and carvacrol (individually, as well as in different combination) showed that they increased the sensitivity of Salmonella enterica serovar Typhimurium DT104 towards five antibiotics [146].
Although the essential oils (EOs) of Chrysanthemum coronarium L. have no activity against Escherichia coli, Staphylococcus aureus or Pseudomonas aeruginosa, its application together with ticarcillin, imipenem, gentamicin and tobramycin in culture media results in a decrease in the antimicrobial activity of these antibiotics against most bacteria tested;, but a synergistic effect was also detected against some other bacteria [147,148]. Furthermore, as antimicrobial resistance in Salmonella enterica serovar Typhimurium DT104 is attributed to the interaction of antibiotic transporters with the efflux system AcrAB–TolC, it becomes speculative to think that the modulation of EPs by PDSs makes pathogenic bacteria sensitive towards antibiotics.
5.4. Biofilms Inhibition
Biofilms are an assembly of surface-integrated microbial populations enclosing an exopolysaccharide matrix [149]. Biofilm formation is a worrisome situation both in hospital environments and the food industry [150,151,152,153,154]. Comprehensive research investigating alternative plans to stop microbial biofilm formation has emphasized on the effectiveness of PDSs in limiting biofilm formation by important pathogens like Listeria monocytogenes [155], Staphylococcus aureus [156,157,158,159,160], Pseudomonas aeruginosa [161,162], Escherichia coli [163,164] and Klebsiella pneumoniae. PDSs, at sub-inhibitory concentrations, cause changes in the transcription of genes critical for biofilm formation, thereby contributing to antibiofilm activity [165,166,167,168,169,170]. In a study, Brackman and coworkers (2008) reported inhibitory effects of trans-cinnamaldehyde on biofilm formation among Vibrio spp. [171]. Although trans-cinnamaldehyde is competent enough to diminish autoinducer-2-dependent quorum sensing and biofilm production through the transcriptional regulation of specific genes, comparable transcription modulatory effects without disturbing bacterial growth have been seen in Salmonella [172] and Pseudomonas aeruginosa [173]. Trans-cinnamaldehyde also controls biofilm development and halts completely grown biofilms of Cronobacter sakazakii, found on stainless steel surfaces and feeding bottles and uropathogenic Escherichia coli present in urinary catheters [165,174]. Equally, terpenes like carvacrol, geraniol, thymol and EOs, exhibit potent antibiofilm activity against diverse bacterial and fungal biofilms [175,176,177]. As quorum sensing regulates the expression of genes associated with the production of virulence factors [178,179,180], focus has been given to PDSs that interfere with cell-to-cell communication, as anti-quorum-sensing molecules, which cause a decrease in the expression of virulence genes [181,182,183]. For example, the expression of luxR (a transcriptional regulator of quorum sensing) in C. sakazakii shows a decreasing trend in response to application of trans-cinnamaldehyde [165]. Additionally, Bodini and coworkers (2009) reported the antiquorum sensing properties of p-coumaric acid and garlic extracts [184]. Moreover, goldenseal, a PDS from Hydrastis canadensis L., disrupts microbial quorum sensing activity by interfering with the AgrCA two-component system in MRSA [185]. Streptococcus pneumoniae forms orderly biofilms that show persistence in human nasopharynx, permitting the development of diseases, such as pneumonia, otitis media, bacteremia and meningitis. Treating Streptococcus pneumoniae with 220D-F2, a plant extract from Rubus ulmifolius, in combination with ellagic acid and its derivatives showed a dose-dependent inhibition of biofilm formation [186]. Studies on bacterial viability following treatment with 100–200 mg/mL of 220D-F2 showed bactericidal effects as early as 3 h post inoculation for Streptococcus pneumoniae. Minimum inhibitory concentration (MIC) studies revealed that 80 mg/mL of 220D-F2 eliminates antibiotic resistant pneumococci strains. Testing essential oils from different plant sources, such as Origanum vulgare, Mentha piperita and Hofmeisteria schaffneri, against S. aureus has shown significant inhibitory activity. Studies on the antibiofilm activity of EOs and solvent extracts of different plants, such as Mentha piperita, Pimpinella anisum and Coriandrum sativum, revealed interference with the attachment of bacteria (Staphylococcus aureus, Escherichia coli and others) to the host tissues [187]. A potent antibiofilm activity against Staphylococcus aureus and Escherichia coli was observed for coriander oil at concentrations of 0.8 and 1.6 mg/mL. Non-accountable for the rise in antimicrobial resistance, carvacrol exerts an inhibitory effect, through interference with biofilm formation, against Staphylococcus aureus and Staphylococcus epidermidis [156,167]. Essential oils from Citrus sinensis, Citrus bergamia, Sideritis erythrantha and Eucalyptus globulus were found to be effective in combating vancomycin-resistant enterococci (VRE) [188,189,190,191]. In addition, a study on the antibiofilm activity of the methanolic extracts of Sclero caryabirrea showed that, besides inhibiting the swarming capability of Pseudomonas aeruginosa, they disturb quorum-sensing-mediated biofilm formation. Moreover, the extracts also exerted regulatory effects on the secretion of pyoverdine and proteases (quorum sensing reliant pathogenic factors). Growth and biofilm inhibitory effects on Staphylococcus aureus have also been observed for extracts obtained from Quercus cerris [192]. Staphylococcus aureus pathogenicity ranges from localized skin infection to systemic infection of different tissues. Finally, an antibiofilm activity of flavonoids from Vaccinium macrocarpon(cranberry) was identified against Enterococcus faecalis, Escherichia coli and Pseudomonas aeruginosa [193].
5.5. Attenuating Bacterial Virulence
The production of virulence determinants, like capsule polysaccharides, plays an important role in the development of virulence [49] in bacteria like S. pneumoniae [16,194,195] Staphylococcus aureus [196], Klebsiella pneumoniae and Bacillus anthracis. The capsule protects bacteria from phagocytosis [194]; thus, improving bacterial growth within the host [196]. Apart from virulence, the existence of a capsule helps in biofilm production and adhesion [197,198]. Capsule formation can lead to pathology even in plants; capsular polysaccharides of Pseudomonas solanacearum, for example, occlude xylem vessels of plants eventually causing the plant’s death. Foodborne pathogens adhere to the intestinal epithelium and invade through a receptor-mediated mechanism involving the surface proteins InlA and InlB; the presence of PDSs was found to decrease virulence factor production in Listeria monocytogenes [169], uropathogenic Escherichia coli [165] and Salmonella enterica serovar Enteritidis [199], reducing their invasion capacity. Additionally, capsular polysaccharides, necessary for pathogen survival inside the host, showed a reduction following exposure to PDSs [200]. Furthermore, various derivatives of salicylic acid, which is a signal molecule involved in plant protection [201], including sodium salicylate, bismuth subsalicylate and bismuth dimercaprol [202], were shown to regulate the construction of bacterial capsules. Further studies on these derivatives revealed that salicylic acid is efficient in reducing capsule manufacturing by regulating the expression of bacterial regulators of the capsule synthesis in Staphylococcus aureus. As quorum sensing, adhesion and capsular polysaccharides play a vital role for interaction among microbes and flourishment within the host, it becomes imperative to exploit them for therapeutics for overcoming the burden of increasing antibiotic resistance among microbes.
6. Hurdles to Overcome
Regardless of their antimicrobial effects, the use of PDSs as alternatives or complements (either for increasing the activity or decreasing the dose) to antibiotics is impeded by regulatory factors that pose a challenge regarding their implementation in the treatment of diseases. One challenge is the development of effective extraction and purification systems for the isolation of newer and safer plant-derived antimicrobials [203]. In addition to solvent extractions systems, microwave and ultrasonic-assisted extraction methods are currently being explored for the extraction of PDSs [204]. Screening bioactive compounds for elucidating their structure, function and action mechanisms is necessary to fine tune their production and maximize their performance with simplicity, specificity and speed. Isolation and purification processes have been accelerated with the development of high-pressure liquid chromatography (HPLC) and a variety of spectroscopic techniques (UV-visible, infrared (IR), nuclear magnetic resonance (NMR) and mass spectroscopy [205]. Additionally, reduction in the production cost can be achieved by rationalizing their chemical synthesis and further increasing their efficacy by modifying their structures. To properly address the feasibility of the use of PSDs, the following points need to be considered: (1) dose in terms of bacteriostatic/-cidal effect; (2) variation in phytochemical constituents; (3) safety in humans; and (4) efficacy without any concurrent effect in terms of resistance development. A deeper insight into the toxicity of PDSs, their mechanism of action and influential interactions with antibiotics needs further exploration to have a complete understanding of their role in disease treatment and for their broader adoption in the near future. A recent strategic approach, using fluorescently labeled antibiotics, promises good results in resistance and toxicological studies and in elucidating the potency of PDSs in the treatment of different diseases [206]. Though much attention has been paid to adding flexibility to approval strategies, consistency in safety, efficacy and delivery methods is required for their commercialized use against broad range of pathogens.
7. Conclusions
The phenomenon of antibiotic resistance is not only old but is also a very difficult situation that emerges among bacteria exposed to antibiotics. Sadly, it has not been given a priority even though it has become a serious issue concerning human health. This worrisome situation is continuously growing, but still, it has not been included in planning strategies among health experts, the scientific community or researchers. The current crisis of rising multidrug resistance in bacteria is creating a global threat, necessitating the development of new alternatives. With the relative absence of new antimicrobials in the market, the sleeping giants of the pharmaceutical industry (medicinal herbs) have high potential for being a source of natural drugs that can be utilized to combat the menace created by antibiotic resistance. Plant-based antimicrobials have a potential for use in the manufacturing of drugs. Exhibiting a potent antimicrobial activity, plant-based antimicrobials, either alone or combined with antibiotics, can help in dealing with the present crisis of antibiotic resistance. A study by Carradori et al. shows antimicrobial activity of Crocus sativus-derived compounds against different bacterial sp. [207]. This study shows the promising antimicrobial activity from the modified compounds of Crocus sativus. The MIC values obtained in a particular activity were showing synergy among Crocus sativus components. These kinds of studies provide an insight that certain chemical and structural modifications can provide us with more promising and effective compounds that can help in the formulation of innovative drugs to fight against antifungal and antimicrobial situation. In conclusion, there is an urgent requirement to develop plant-based antimicrobials to counteract antibiotic resistance. The search for natural compounds, extracted from medicinal plants, is a necessity. Research based on ethnopharmacology the field of study that uses plant herbs as a therapeutic option, may be used as a guide in our search for suitable agents against the spread of antibiotic resistance. The global nature of this crisis and its substantial health and economic burdens prompt us to urgently identify new alternatives as well as implement new policies to combat antibiotic resistance.
Acknowledgments
The authors are grateful to the support provided by King Saud University, Riyadh, Saudi Arabia, Indian Council of Medical Research (ICMR), India for fellowship to I.S., and University Grants Commission (UGC), India for startup grant to A.T.J.
Author Contributions
A.T.J. and Q.M.R.H. conceived the idea. H.M.A.A., I.S. and V.K. contributed to the writing of the manuscript. H.A.-S. contributed to review of literature, A.T.J. and I.A.R. edited the manuscript and design figures. A.T.J. and Q.M.R.H. reviewed and approved the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
No funding was availed for the study.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.WHO . World Health Organization Model List of Essential Medicines. WHO; Geneva, Switzerland: Mar, 2017. amended August 2017; 20th List. [Google Scholar]
- 2.Dunphy L.J., Yen P., Papin J.A. Integrated experimental and computational analyses reveal differential metabolic functionality in antibiotic-resistant Pseudomonas aeruginosa. Cell Syst. 2019;8:3–14. doi: 10.1016/j.cels.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoiby N., Bjarnsholt T., Givskov M., Molin S., Ciofu O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents. 2010;35:322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Ioannidis A., Magana M., Bologa C.G., Oprea T.I., Paulsen I.T., Tegos G.P. Defining the microbial effluxome in the content of the host-microbiome interaction. Front. Pharm. 2015;6:31. doi: 10.3389/fphar.2015.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lister P.D., Wolter D.J., Hanson N.D. Antibacterial-resistant Pseudomonas aeruginosa: Clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin. Microbiol. Rev. 2009;22:582–610. doi: 10.1128/CMR.00040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pang Z., Raudonis R., Glick B.R., Lin T.J., Cheng Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019;37:177–192. doi: 10.1016/j.biotechadv.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Reygaert W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018;4:482–501. doi: 10.3934/microbiol.2018.3.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beceiro A., Tomas M., Bou G. Antimicrobial resistance and virulence: A successful or deleterious association in the bacterial world? Clin. Microbiol. Rev. 2013;26:185–230. doi: 10.1128/CMR.00059-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beighton D. Can the ecology of the dental biofilm be beneficially altered? Adv. Dent. Res. 2009;21:69–73. doi: 10.1177/0895937409335641. [DOI] [PubMed] [Google Scholar]
- 10.Bunce J.T., Hellyer P. Antibiotic resistance and antibiotic prescribing by dentists in England 2007–2016. Br. Dent. J. 2018;225:81–84. doi: 10.1038/sj.bdj.2018.525. [DOI] [PubMed] [Google Scholar]
- 11.Guerrini L., Monaco A., Pietropaoli D., Ortu E., Giannoni M., Marci M.C. Antibiotics in dentistry: A narrative review of literature and guidelines considering antibiotic resistance. Open Dent. J. 2019;13:383–398. doi: 10.2174/1874210601913010383. [DOI] [Google Scholar]
- 12.Bansal R., Jain A., Goyal M., Singh T., Sood H., Malviya H.S. Antibiotic abuse during endodontic treatment: A contributing factor to antibiotic resistance. J. Fam. Med. Prim. Care. 2019;8:3518–3524. doi: 10.4103/jfmpc.jfmpc_768_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haque M., Sartelli M., Haque S.Z. Dental infection and resistance-global health consequences. Dent. J. 2019;7:22. doi: 10.3390/dj7010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu W., Zhou Y., Li Z., Huang T., Xiao Y., Cheng L., Peng X., Zhang L., Ren B. Application of antibiotics/antimicrobial agents on dental caries. Biomed. Res. Int. 2020;2020:5658212. doi: 10.1155/2020/5658212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spellberg B., Powers J.H., Brass E.P., Miller L.G., Edwards J.E.J. Trends in antimicrobial drug development: Implications for the future. Clin. Infect. Dis. 2004;38:1279–1286. doi: 10.1086/420937. [DOI] [PubMed] [Google Scholar]
- 16.Yother J. Capsules of Streptococcus pneumoniae and other bacteria: Paradigms for polysaccharide biosynthesis and regulation. Annu. Rev. Microbiol. 2011;65:563–581. doi: 10.1146/annurev.micro.62.081307.162944. [DOI] [PubMed] [Google Scholar]
- 17.Lewis K., Ausubel F.M. Prospects for plant-derived antibacterials. Nat. Biotechnol. 2006;24:1504–1507. doi: 10.1038/nbt1206-1504. [DOI] [PubMed] [Google Scholar]
- 18.Kolli M.E., Laouer H., Kolli H.E., Akkal S., Sahli F. Chemical analysis, antimicrobial and anti-oxidative properties of Daucus gracilis essential oil and its mechanism of action. Asian Pac. J. Trop. Biomed. 2016;6:8–15. doi: 10.1016/j.apjtb.2015.08.004. [DOI] [Google Scholar]
- 19.Gemeda N., Tadele A., Lemma H., Girma B., Addis G., Tesfaye B., Abebe A., Gemechu W., Yirsaw K., Teka F., et al. Development, characterization, and evaluation of novel broad-spectrum antimicrobial topical formulations from Cymbopogon martini (Roxb.) W. watson essential oil. Evid. Based Complement. Altern. Med. 2018;2018:9812093. doi: 10.1155/2018/9812093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Appendino G., Scafati O.T., Romano A., Pollastro F., Avonto C., Rubiolo P. Genepolide, a sesterpene gamma-lactone with a novel carbon skeleton from mountain wormwood (Artemisia umbelliformis) J. Nat. Prod. 2009;72:340–344. doi: 10.1021/np800468m. [DOI] [PubMed] [Google Scholar]
- 21.Conrad J., Dinchev D., Klaiber I., Mika S., Kostova I., Kraus W. A novel furostanol saponin from Tribulus terrestris of bulgarian origin. Fitoterapia. 2004;75:117–122. doi: 10.1016/j.fitote.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Deng X.H., Fang F.F., Zheng C.J., Wu Y., Qin L.P. Monoterpenoids from the whole herb of Veronicastrum axillare. Pharm. Biol. 2014;52:661–663. doi: 10.3109/13880209.2013.863947. [DOI] [PubMed] [Google Scholar]
- 23.Gao L., Zhang L., Li N., Liu J.Y., Cai P.L., Yang S.L. New triterpenoid saponins from Patrinia scabiosaefolia. Carbohydr. Res. 2011;346:2881–2885. doi: 10.1016/j.carres.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Guo Y., Liu Y.X., Kang L.P., Zhang T., Yu H.S., Zhao Y., Xiong C.Q., Ma B.P. Two novel furostanol saponins from the tubers of Ophiopogon japonicus. J. Asian Nat. Prod. Res. 2013;15:459–465. doi: 10.1080/10286020.2013.783024. [DOI] [PubMed] [Google Scholar]
- 25.Liu S., Hsieh D., Yang Y.L., Xu Z., Peto C., Jablons D.M., You L. Coumestrol from the national cancer institute’s natural product library is a novel inhibitor of protein kinase CK2. BMC Pharm. Toxicol. 2013;14:36. doi: 10.1186/2050-6511-14-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramasamy D., Saraswathy A. Vitiquinolone—A quinolone alkaloid from Hibiscus vitifolius linn. Food Chem. 2014;145:970–975. doi: 10.1016/j.foodchem.2013.08.128. [DOI] [PubMed] [Google Scholar]
- 27.Su Y.F., Zhang Z.X., Guo C.Y., Guo D.A. A nobel cyanogenic glycoside from Semiaquilegia adoxoides. J. Asian Nat. Prod. Res. 2005;7:171–174. doi: 10.1080/10286020310001625148. [DOI] [PubMed] [Google Scholar]
- 28.Wiedenfeld H., Dumaa M., Malinowski M., Furmanowa M., Narantuya S. Phytochemical and analytical studies of extracts from Rhodiola rosea and Rhodiola quadrifida. Pharmazie. 2007;62:308–311. [PubMed] [Google Scholar]
- 29.Agarwal A., D’Souza P., Johnson T.S., Dethe S.M., Chandrasekaran C. Use of in vitro bioassays for assessing botanicals. Curr. Opin. Biotechnol. 2014;25:39–44. doi: 10.1016/j.copbio.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 30.Pathmasiri W., Seedi H.R.E., Han X., Janson J.C., Huss U., Bohlin L. Aryl ketones from Acronychia pedunculata with cyclooxygenase-2 inhibitory effects. Chem. Biodivers. 2005;2:463–469. doi: 10.1002/cbdv.200590026. [DOI] [PubMed] [Google Scholar]
- 31.Xiao Z.Y., Mu Q., Shiu W.K., Zeng Y.H., Gibbons S. Polyisoprenylated benzoylphloroglucinol derivatives from Hypericum sampsonii. J. Nat. Prod. 2007;70:1779–1782. doi: 10.1021/np0704147. [DOI] [PubMed] [Google Scholar]
- 32.Zhang H.J., Tamez P.A., Vu D.H., Ghee T.T., Nguyen V.H., Le T.X., Le M.H., Nguyen M.C., Do T.T., Soejarto D.D., et al. Antimalarial compounds from Rhaphidophora decursiva. J. Nat. Prod. 2001;64:772–777. doi: 10.1021/np010037c. [DOI] [PubMed] [Google Scholar]
- 33.Atanasov A.G., Blunder M., Fakhrudin N., Liu X., Noha S.M., Malainer C., Kramer M.P., Cocic A., Kunert O., Schinkovitz A., et al. Polyacetylenes from notopterygium incisum--new selective partial agonists of peroxisome proliferator-activated receptor-gamma. PLoS ONE. 2013;8:e61755. doi: 10.1371/journal.pone.0061755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fakhrudin N., Waltenberger B., Cabaravdic M., Atanasov A.G., Malainer C., Schachner D., Heiss E.H., Liu R., Noha S.M., Grzywacz A.M., et al. Identification of plumericin as a potent new inhibitor of the NF-kappaB pathway with anti-inflammatory activity in vitro and in vivo. Br. J. Pharm. 2014;171:1676–1686. doi: 10.1111/bph.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mabona U., Viljoen A., Shikanga E., Marston A., Vuuren S.V. Antimicrobial activity of southern African medicinal plants with dermatological relevance: From an ethnopharmacological screening approach, to combination studies and the isolation of a bioactive compound. J. Ethnopharmacol. 2013;148:45–55. doi: 10.1016/j.jep.2013.03.056. [DOI] [PubMed] [Google Scholar]
- 36.Nievergelt A., Huonker P., Schoop R., Altmann K.H., Gertsch J. Identification of serotonin 5-HT1A receptor partial agonists in ginger. Bioorg. Med. Chem. 2010;18:3345–3351. doi: 10.1016/j.bmc.2010.02.062. [DOI] [PubMed] [Google Scholar]
- 37.Junio H.A., Cordero A.A.S., Ettefagh K.A., Burns J.T., Micko K.T., Graf T.N., Richter S.J., Cannon R.E., Oberlies N.H., Cech N.B. Synergy-directed fractionation of botanical medicines: A case study with goldenseal (Hydrastis canadensis) J. Nat. Prod. 2011;74:1621–1629. doi: 10.1021/np200336g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ndhlala A.R., Aderogba M.A., Ncube B., Staden J.V. Anti-oxidative and cholinesterase inhibitory effects of leaf extracts and their isolated compounds from two closely related Croton species. Molecules. 2013;18:1916–1932. doi: 10.3390/molecules18021916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nitteranon V., Zhang G., Darien B.J., Parkin K. Isolation and synergism of in vitro anti-inflammatory and quinone reductase (QR) inducing agents from the fruits of Morinda citrifolia (noni) Food Res. Int. 2011;44:2271–2277. doi: 10.1016/j.foodres.2010.11.009. [DOI] [Google Scholar]
- 40.Tafesh A., Najami N., Jadoun J., Halahlih F., Riepl H., Azaizeh H. Synergistic antibacterial effects of polyphenolic compounds from olive mill wastewater. Evid. Based Complement. Alternat. Med. 2011;2011:1–9. doi: 10.1155/2011/431021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang F., Zhao S., Li F., Zhang B., Qu Y., Sun T., Luo T., Li D. Investigation of antioxidant interactions between Radix astragali and Cimicifuga foetida and identification of synergistic antioxidant compounds. PLoS ONE. 2014;9:e87221. doi: 10.1371/journal.pone.0087221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goretta L.A., Leveques A., Giuffrida F., Michailidis F.R., Viton F., Barron D., Paton M.D., Manzano S.G., Buelga C.S., Williamson G., et al. Elucidation of (-)-epicatechin metabolites after ingestion of chocolate by healthy humans. Free. Radic. Biol. Med. 2012;53:787–795. doi: 10.1016/j.freeradbiomed.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 43.Akao T., Yoshino T., Kobashi K., Hattori M. Evaluation of salicin as an antipyretic prodrug that does not cause gastric injury. Planta Med. 2002;68:714–718. doi: 10.1055/s-2002-33792. [DOI] [PubMed] [Google Scholar]
- 44.Atkinson C., Berman S., Humbert O., Lampe J.W. In vitro incubation of human feces with daidzein and antibiotics suggests interindividual differences in the bacteria responsible for equol production. J. Nutr. 2004;134:596–599. doi: 10.1093/jn/134.3.596. [DOI] [PubMed] [Google Scholar]
- 45.Chen J., Ma X., Gao K., Wang Y., Zhao H., Wu H., Wang J., Xie H., OuYang Y., Luo L., et al. The active ingredients of Jiang-Zhi-Ning: Study of the nelumbo nucifera alkaloids and their main bioactive metabolites. Molecules. 2012;17:9855–9867. doi: 10.3390/molecules17089855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guerrero L., Margalef M., Pons Z., Quinones M., Arola L., Arnal A.A., Muguerza B. Serum metabolites of proanthocyanidin-administered rats decrease lipid synthesis in HepG2 cells. J. Nutr. Biochem. 2013;24:2092–2099. doi: 10.1016/j.jnutbio.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 47.Ling Y., Chen M., Wang K., Sun Z., Li Z., Wu B., Huang C. Systematic screening and characterization of the major bioactive components of poria cocos and their metabolites in rats by LC-ESI-MS(n) Biomed. Chromatogr. 2012;26:1109–1117. doi: 10.1002/bmc.1756. [DOI] [PubMed] [Google Scholar]
- 48.Wan J.Y., Liu P., Wang H.Y., Qi L.W., Wang C.Z., Li P., Yuan C.S. Biotransformation and metabolic profile of American ginseng saponins with human intestinal microflora by liquid chromatography quadrupole time-of-flight mass spectrometry. J. Chromatogr. A. 2013;1286:83–92. doi: 10.1016/j.chroma.2013.02.053. [DOI] [PubMed] [Google Scholar]
- 49.Xie C., Kang J., Chen J.R., Nagarajan S., Badger T.M., Wu X. Phenolic acids are in vivo atheroprotective compounds appearing in the serum of rats after blueberry consumption. J. Agric. Food Chem. 2011;59:10381–10387. doi: 10.1021/jf2025264. [DOI] [PubMed] [Google Scholar]
- 50.Tawfike A.F., Viegelmann C., Ebel R.E. Metabolomics and dereplication strategies in natural products. Methods Mol. Biol. 2013;1055:227–244. doi: 10.1007/978-1-62703-577-4_17. [DOI] [PubMed] [Google Scholar]
- 51.Wolfender J.L., Marti G., Thomas A., Bertrand S. Current approaches and challenges for the metabolite profiling of complex natural extracts. J. Chromatogr. A. 2015;1382:136–164. doi: 10.1016/j.chroma.2014.10.091. [DOI] [PubMed] [Google Scholar]
- 52.Gülcemal D., Masullo M., Napolitano A., Karayıldırım T., Bedir E., Çalışkan Ö.A., Piacente S. Oleanane glycosides from Astragalus tauricolus: Isolation and structural elucidation based on a preliminary liquid chromatography-electrospray ionization tandem mass spectrometry profiling. Phytochemistry. 2013;86:184–194. doi: 10.1016/j.phytochem.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 53.Hou C.C., Chen C.H., Yang N.S., Chen Y.P., Lo C.P., Wang S.Y., Tien Y.J., Tsai P.W., Shyur L.F. Comparative metabolomics approach coupled with cell- and gene-based assays for species classification and anti-inflammatory bioactivity validation of echinacea plants. J. Nutr. Biochem. 2010;21:1045–1059. doi: 10.1016/j.jnutbio.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 54.Inui T., Wang Y., Pro S.M., Franzblau S.G., Pauli G.F. Unbiased evaluation of bioactive secondary metabolites in complex matrices. Fitoterapia. 2012;83:1218–1225. doi: 10.1016/j.fitote.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keerthi D., Geethu C., Nair R.A., Pillai P. Metabolic profiling of zingiber zerumbet following pythium myriotylum infection: Investigations on the defensive role of the principal secondary metabolite, zerumbone. Appl. Biochem. Biotechnol. 2014;172:2593–2603. doi: 10.1007/s12010-013-0707-z. [DOI] [PubMed] [Google Scholar]
- 56.Mao Q., Yang J., Cui X.M., Li J.J., Qi Y.T., Zhang P.H., Wang Q. Target separation of a new anti-tumor saponin and metabolic profiling of leaves of panax notoginseng by liquid chromatography with eletrospray ionization quadrupole time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2012;59:67–77. doi: 10.1016/j.jpba.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 57.Modarai M., Yang M., Suter A., Kortenkamp A., Heinrich M. Metabolomic profiling of liquid echinacea medicinal products with in vitro inhibitory effects on cytochrome P450 3A4 (CYP3A4) Planta Med. 2010;76:378–385. doi: 10.1055/s-0029-1186152. [DOI] [PubMed] [Google Scholar]
- 58.Sandasi M., Kamatou G.P., Viljoen A.M. An untargeted metabolomic approach in the chemotaxonomic assessment of two salvia species as a potential source of alpha-bisabolol. Phytochemistry. 2012;84:94–101. doi: 10.1016/j.phytochem.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 59.Gyllenhaal C., Kadushin M.R., Southavong B., Sydara K., Bouamanivong S., Xaiveu M., Xuan L.T., Hiep N.T., Hung N.V., Loc P.K., et al. Ethnobotanical approach versus random approach in the search for new bioactive compounds: Support of a hypothesis. Pharm. Biol. 2012;50:30–41. doi: 10.3109/13880209.2011.634424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khafagi I.K., Dewedar A. The efficiency of random versus ethno-directed research in the evaluation of Sinai medicinal plants for bioactive compounds. J. Ethnopharmacol. 2000;71:365–376. doi: 10.1016/S0378-8741(00)00164-1. [DOI] [PubMed] [Google Scholar]
- 61.Nielsen A.L., Kristensen L.H., Stephansen K.B., Kristensen J.B., Helgstrand C., Lees M., Cloos P., Helin K., Gajhede M., Olsen L. Identification of catechols as histone-lysine demethylase inhibitors. FEBS Lett. 2012;586:1190–1194. doi: 10.1016/j.febslet.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 62.Oliveira D.R., Leitão G.G., Coelho T.S., Silva P.E.A., Lourenço M.C.S., Leitão S.G. Ethnopharmacological versus random plant selection methods for the evaluation of the antimycobacterial activity. Rev. Bras. Farm. 2011;21:793–806. doi: 10.1590/S0102-695X2011005000084. [DOI] [Google Scholar]
- 63.Shaneyfelt M.E., Burke A.D., Graff J.W., Jutila M.A., Hardy M.E. Natural products that reduce rotavirus infectivity identified by a cell-based moderate-throughput screening assay. Virol. J. 2006;3:68. doi: 10.1186/1743-422X-3-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Atanasov A.G., Waltenberger B., Wenzig E.M.P., Linder T., Wawrosch C., Uhrin P., Temml V., Wang L., Schwaiger S., Heiss E.H., et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015;33:1582–1614. doi: 10.1016/j.biotechadv.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ekuadzi E., Dickson R., Fleischer T., Annan K., Pistorius D., Oberer L., Gibbons S. Flavonoid glycosides from the stem bark of margaritaria discoidea demonstrate antibacterial and free radical scavenging activities. Phytother. Res. 2014;28:784–787. doi: 10.1002/ptr.5053. [DOI] [PubMed] [Google Scholar]
- 66.Noreen Y., Seedi H.E., Perera P., Bohlin L. Two new isoflavones from ceiba pentandra and their effect on cyclooxygenase-catalyzed prostaglandin biosynthesis. J. Nat. Prod. 1998;61:8–12. doi: 10.1021/np970198+. [DOI] [PubMed] [Google Scholar]
- 67.Siriwatanametanon N., Heinrich M. The Thai medicinal plant gynura pseudochina var. hispida: Chemical composition and in vitro NF-kappaB inhibitory activity. Nat. Prod. Commun. 2011;6:627–630. doi: 10.1177/1934578X1100600512. [DOI] [PubMed] [Google Scholar]
- 68.Fabricant D.S., Farnsworth N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001;109:69–75. doi: 10.1289/ehp.01109s169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heinrich M. Ethnopharmacology in the 21st century—Grand challenges. Front. Pharm. 2010;1:8. doi: 10.3389/fphar.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leonti M. The future is written: Impact of scripts on the cognition, selection, knowledge and transmission of medicinal plant use and its implications for ethnobotany and ethnopharmacology. J. Ethnopharmacol. 2011;134:542–555. doi: 10.1016/j.jep.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 71.Ahamad S., Rahman S., Khan F.I., Dwivedi N., Ali S., Kim J., Imtaiyaz Hassan M. QSAR based therapeutic management of M. tuberculosis. Arch. Pharm. Res. 2017;40:676–694. doi: 10.1007/s12272-017-0914-1. [DOI] [PubMed] [Google Scholar]
- 72.Gavernet L., Talevi A., Castro E.A., Blanch L.E.B. A Combined virtual screening 2D and 3D QSAR methodology for the selection of new anticonvulsant candidates from a natural product library. QSAR Comb. Sci. 2008;27:1120–1129. doi: 10.1002/qsar.200730055. [DOI] [Google Scholar]
- 73.Gaulton A., Bellis L.J., Bento A.P., Chambers J., Davies M., Hersey A., Light Y., McGlinchey S., Michalovich D., Lazikani B.A., et al. ChEMBL: A large-scale bioactivity database for drug discovery. Nucleic Acids Res. 2012;40 doi: 10.1093/nar/gkr777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li Q., Cheng T., Wang Y., Bryant S.H. PubChem as a public resource for drug discovery. Drug Discov. Today. 2010;15:1052–1057. doi: 10.1016/j.drudis.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fakhrudin N., Ladurner A., Atanasov A.G., Heiss E.H., Baumgartner L., Markt P., Schuster D., Ellmerer E.P., Wolber G., Rollinger J.M., et al. Computer-aided discovery, validation, and mechanistic characterization of novel neolignan activators of peroxisome proliferator-activated receptor gamma. Mol. Pharm. 2010;77:559–566. doi: 10.1124/mol.109.062141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang H., Xu X., Chen L., Chen J., Hu L., Jiang H., Shen X. Molecular determinants of magnolol targeting both RXRalpha and PPARgamma. PLoS ONE. 2011;6:e28253. doi: 10.1371/journal.pone.0028253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Waszkowycz B., Clark D.E., Gancia E. Outstanding challenges in protein–ligand docking and structure-based virtual screening. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2011;1:229–259. doi: 10.1002/wcms.18. [DOI] [Google Scholar]
- 78.Balamurugan K., Nishanthini A., Mohan V.R. GC–MS analysis of Polycarpaea corymbosa (L.) lam whole plant. Asian Pac. J. Trop. Biomed. 2012;2:S1289–S1292. doi: 10.1016/S2221-1691(12)60402-X. [DOI] [Google Scholar]
- 79.Doshi G.M., Nalawade V.V., Mukadam A.S., Chaskar P.K., Zine S.P., Somani R.R., Une H.D. Structural elucidation of chemical constituents from Benincasa hispida seeds and carissa congesta roots by gas chromatography: Mass spectroscopy. Pharmacogn. Res. 2015;7:282–293. doi: 10.4103/0974-8490.157179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dubey D., Patnaik R., Ghosh G., Padhy R.N. In vitro antibacterial activity, gas chromatography-mass spectrometry analysis of Woodfordia fruticosa kurz. leaf extract and host toxicity testing with in vitro cultured lymphocytes from human umbilical cord blood. Osong Public Health Res. Perspect. 2014;5:298–312. doi: 10.1016/j.phrp.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mickymaray S., Aboody M.S.A., Rath P.K., Annamalai P., Nooruddin T. Screening and antibacterial efficacy of selected Indian medicinal plants. Asian Pac. J. Trop. Biomed. 2016;6:185–191. doi: 10.1016/j.apjtb.2015.12.005. [DOI] [Google Scholar]
- 82.Sabatini S., Piccioni M., Felicetti T., De Marco S., Manfroni G., Pagiotti R., Nocchetti M., Cecchetti V., Pietrella D. Investigation on the effect of known potent S. aureus NorA efflux pump inhibitors on the staphylococcal biofilm formation. RSC Adv. 2017;7:37007–37014. doi: 10.1039/C7RA03859C. [DOI] [Google Scholar]
- 83.Mishra M.P., Rath S., Swain S.S., Ghosh G., Das D., Padhy R.N. In vitro antibacterial activity of crude extracts of 9 selected medicinal plants against UTI causing MDR bacteria. J. King Saud Univ. Sci. 2017;29:84–95. doi: 10.1016/j.jksus.2015.05.007. [DOI] [Google Scholar]
- 84.Cesa S., Sisto F., Zengin G., Scaccabarozzi D., Kokolakis A.K., Scaltrito M.M., Grande R., Locatelli M., Cacciagrano F., Angiolella L. Phytochemical analyses and pharmacological screening of neem oil. S. Afr. J. Bot. 2019;120:331–337. doi: 10.1016/j.sajb.2018.10.019. [DOI] [Google Scholar]
- 85.Semeniuc C.A., Pop C.R., Rotar A.M. Antibacterial activity and interactions of plant essential oil combinations against gram-positive and gram-negative bacteria. J. Food Drug Anal. 2017;25:403–408. doi: 10.1016/j.jfda.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zengin G., Menghini L., Di Sotto A., Mancinelli R., Sisto F., Carradori S., Cesa S., Fraschetti C., Filippi A., Angiolella L., et al. Chromatographic Analyses, in vitro biological activities, and cytotoxicity of cannabis sativa L. essential oil: A multidisciplinary study. Molecules. 2018;23:3266. doi: 10.3390/molecules23123266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ruiz G., Turner T., Nelson E., Sparks L., Langland J. Bacterial development of resistance to botanical antimicrobials. J. Evol. Health. 2017;2:3. doi: 10.15310/2334-3591.1065. [DOI] [Google Scholar]
- 88.Roy S.K., Pahwa S., Nandanwar H., Jachak S.M. Phenylpropanoids of Alpinia galanga as efflux pump inhibitors in mycobacterium smegmatis mc(2) 155. Fitoterapia. 2012;83:1248–1255. doi: 10.1016/j.fitote.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 89.Dwivedi G.R., Upadhyay H.C., Yadav D.K., Singh V., Srivastava S.K., Khan F., Darmwal N.S., Darokar M.P. 4-Hydroxy-alpha-tetralone and its derivative as drug resistance reversal agents in multi drug resistant Escherichia coli. Chem. Biol. Drug Des. 2014;83:482–492. doi: 10.1111/cbdd.12263. [DOI] [PubMed] [Google Scholar]
- 90.Aghayan S.S., Mogadam H.K., Fazli M., Sarokhalil D.D., Khoramrooz S.S., Jabalameli F., Yaslianifard S., Mirzaii M. The effects of berberine and palmatine on efflux pumps inhibition with different gene patterns in Pseudomonas aeruginosa isolated from burn infections. Avicenna J. Med. Biotechnol. 2017;9:2–7. [PMC free article] [PubMed] [Google Scholar]
- 91.Maisuria V.B., Hosseinidoust Z., Tufenkji N. Polyphenolic extract from maple syrup potentiates antibiotic susceptibility and reduces biofilm formation of Pathogenic bacteria. Appl. Environ. Microbiol. 2015;81:3782–3792. doi: 10.1128/AEM.00239-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dwivedi G.R., Tyagi R., Gupta S., Tripathi S., Pati S., Srivastava S.K., Darokar M.P., Sharma A. Antibiotics potentiating potential of catharanthine against superbug Pseudomonas aeruginosa. J. Biomol. Struct. Dyn. 2018;36:1–15. doi: 10.1080/07391102.2017.1413424. [DOI] [PubMed] [Google Scholar]
- 93.Siriyong T., Srimanote P., Chusri S., Yingyongnarongkul B.E., Suaisom C., Tipmanee V., Voravuthikunchai S.P. Conessine as a novel inhibitor of multidrug efflux pump systems in Pseudomonas aeruginosa. BMC Complement. Altern. Med. 2017;17:1–7. doi: 10.1186/s12906-017-1913-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kakarla P., Floyd J., Mukherjee M., Devireddy A.R., Inupakutika M.A., Ranweera I., Kc R., Shrestha U., Cheeti U.R., Willmon T.M., et al. Inhibition of the multidrug efflux pump LmrS from Staphylococcus aureus by cumin spice Cuminum cyminum. Arch. Microbiol. 2017;199:465–474. doi: 10.1007/s00203-016-1314-5. [DOI] [PubMed] [Google Scholar]
- 95.Chovanova R., Mezovska J., Vaverkova S., Mikulasova M. The inhibition the Tet(K) efflux pump of tetracycline resistant Staphylococcus epidermidis by essential oils from three salvia species. Lett. Appl. Microbiol. 2015;61:58–62. doi: 10.1111/lam.12424. [DOI] [PubMed] [Google Scholar]
- 96.Limaverde P.W., Campina F.F., Cunha F.A.B.D., Crispim F.D., Figueredo F.G., Lima L.F., Datiane M.O.-T.C., Matos Y.D., Braga M.F.B.M., Menezes I.R.A., et al. Inhibition of the TetK efflux-pump by the essential oil of chenopodium ambrosioides L. and alpha-terpinene against Staphylococcus aureus IS-58. Food Chem. Toxicol. 2017;109:957–961. doi: 10.1016/j.fct.2017.02.031. [DOI] [PubMed] [Google Scholar]
- 97.Bag A., Chattopadhyay R.R. Efflux-pump inhibitory activity of a gallotannin from terminalia chebula fruit against multidrug-resistant uropathogenic Escherichia coli. Nat. Prod. Res. 2014;28:1280–1283. doi: 10.1080/14786419.2014.895729. [DOI] [PubMed] [Google Scholar]
- 98.Holler J.G., Christensen S.B., Slotved H.C., Rasmussen H.B., Guzman A., Olsen C.E., Petersen B., Molgaard P. Novel inhibitory activity of the Staphylococcus aureus NorA efflux pump by a kaempferol rhamnoside isolated from persea lingue nees. J. Antimicrob. Chemother. 2012;67:1138–1144. doi: 10.1093/jac/dks005. [DOI] [PubMed] [Google Scholar]
- 99.Maurya A., Dwivedi G.R., Darokar M.P., Srivastava S.K. Antibacterial and synergy of clavine alkaloid lysergol and its derivatives against nalidixic acid-resistant Escherichia coli. Chem. Biol. Drug Des. 2013;81:484–490. doi: 10.1111/cbdd.12103. [DOI] [PubMed] [Google Scholar]
- 100.Shiu W.K., Malkinson J.P., Rahman M.M., Curry J., Stapleton P., Gunaratnam M., Neidle S., Mushtaq S., Warner M., Livermore D.M., et al. A new plant-derived antibacterial is an inhibitor of efflux pumps in Staphylococcus aureus. Int. J. Antimicrob. Agents. 2013;42:513–518. doi: 10.1016/j.ijantimicag.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 101.Bame J.R., Graf T.N., Junio H.A., Bussey R.O., Jarmusch S.A., Elimat T.E., Falkinham J.O., Oberlies N.H., Cech R.A., Cech N.B. Sarothrin from Alkanna orientalis is an antimicrobial agent and efflux pump inhibitor. Planta Med. 2013;79:327–329. doi: 10.1055/s-0032-1328259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mocan A., Babota M., Pop A., Fizesan I., Diuzheva A., Locatelli M., Carradori S., Campestre C., Menghini L., Sisea C.R., et al. Chemical constituents and biologic activities of sage species: A comparison between Salvia officinalis L., S. glutinosa L. and S. transsylvanica (Schur ex Griseb. & Schenk) schur. Antioxidants. 2020;9:480. doi: 10.3390/antiox9060480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Luo Z., Liu Y., Zhao B., Tang M., Dong H., Zhang L., Lv B., Wei L. Ex vivo and in situ approaches used to study intestinal absorption. J. Pharm. Toxicol. Methods. 2013;68:208–216. doi: 10.1016/j.vascn.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 104.Teicher B.A. In vivo/ex vivo and in situ assays used in cancer research: A brief review. Toxicol. Pathol. 2009;37:114–122. doi: 10.1177/0192623308329473. [DOI] [PubMed] [Google Scholar]
- 105.Foster J.R., Lund G., Sapelnikova S., Tyrrell D.L., Kneteman N.M. Chimeric rodents with humanized liver: Bridging the preclinical/clinical trial gap in ADME/toxicity studies. Xenobiotica. 2014;44:109–122. doi: 10.3109/00498254.2013.867553. [DOI] [PubMed] [Google Scholar]
- 106.Zambrowicz B.P., Sands A.T. Knockouts model the 100 best-selling drugs--will they model the next 100? Nat. Rev. Drug Discov. 2003;2:38–51. doi: 10.1038/nrd987. [DOI] [PubMed] [Google Scholar]
- 107.Zhang D., Luo G., Ding X., Lu C. Preclinical experimental models of drug metabolism and disposition in drug discovery and development. Acta Pharm. Sin. B. 2012;2:549–561. doi: 10.1016/j.apsb.2012.10.004. [DOI] [Google Scholar]
- 108.Pulak R. Techniques for analysis, sorting, and dispensing of C. elegans on the COPAS flow-sorting system. Methods Mol. Biol. 2006;351:275–286. doi: 10.1385/1-59745-151-7:275. [DOI] [PubMed] [Google Scholar]
- 109.O’Reilly L.P., Luke C.J., Perlmutter D.H., Silverman G.A., Pak S.C.C. elegans in high-throughput drug discovery. Adv. Drug Deliv. Rev. 2014;69–70:247–253. doi: 10.1016/j.addr.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wood A.J., Lo T.W., Zeitler B., Pickle C.S., Ralston E.J., Lee A.H., Amora R., Miller J.C., Leung E., Meng X., et al. Targeted genome editing across species using ZFNs and TALENs. Science. 2011;333:307. doi: 10.1126/science.1207773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cutuli M.A., Petronio G.P., Vergalito F., Magnifico I., Pietrangelo L., Venditti N., Marco R.G. Galleria mellonella as a consolidated in vivo model hosts: New developments in antibacterial strategies and novel drug testing. Virulence. 2019;10:527–541. doi: 10.1080/21505594.2019.1621649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Humann J., Lenz L.L. Bacterial peptidoglycan degrading enzymes and their impact on host muropeptide detection. J. Innate Immun. 2009;1:88–97. doi: 10.1159/000181181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Upadhyay A., Upadhyaya I., Johny A.K., Venkitanarayanan K. Combating pathogenic microorganisms using plant-derived antimicrobials: A minireview of the mechanistic basis. Biomed. Res. Int. 2014;2014:1–18. doi: 10.1155/2014/761741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sun J., Marais J.P., Khoo C., LaPlante K., Vejborg R.M., Givskov M., Nielsen T.T., Seeram N.P., Rowley D.C. Cranberry (Vaccinium macrocarpon) oligosaccharides decrease biofilm formation by uropathogenic Escherichia coli. J. Funct. Foods. 2015;17:235–242. doi: 10.1016/j.jff.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ulrey R.K., Barksdale S.M., Zhou W., Hoek M.L.V. Cranberry proanthocyanidins have anti-biofilm properties against Pseudomonas aeruginosa. BMC Complement. Altern. Med. 2014;14:499. doi: 10.1186/1472-6882-14-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wojnicz D., Goska D.T., Korzekwa K., Kicia M., Hendrich A.B. Study of the impact of cranberry extract on the virulence factors and biofilm formation by Enterococcus faecalis strains isolated from urinary tract infections. Int. J. Food Sci. Nutr. 2016;67:1005–1016. doi: 10.1080/09637486.2016.1211996. [DOI] [PubMed] [Google Scholar]
- 117.Cushnie T.P., Lamb A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents. 2005;26:343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Simoes L.C., Lemos M., Pereira A.M., Abreu A.C., Saavedra M.J., Simoes M. Persister cells in a biofilm treated with a biocide. Biofouling. 2011;27:403–411. doi: 10.1080/08927014.2011.579599. [DOI] [PubMed] [Google Scholar]
- 119.Suarez M., Haenni M., Canarelli S., Fisch F., Chodanowski P., Servis C., Michielin O., Freitag R., Moreillon P., Mermod N. Structure-function characterization and optimization of a plant-derived antibacterial peptide. Antimicrob. Agents Chemother. 2005;49:3847–3857. doi: 10.1128/AAC.49.9.3847-3857.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Giacinto B., Cosentino M., Sakurada T. Aromatherapy: Basic Mechanisms And Evidence Based Clinical Use. CRC Press Taylor Francis Group; Boca Raton, FL, USA: 2016. p. 174. [Google Scholar]
- 121.Willers C., Wentzel J.F., Plessis L.H.D., Gouws C., Hamman J.H. Efflux as a mechanism of antimicrobial drug resistance in clinical relevant microorganisms: The role of efflux inhibitors. Expert Opin. Targets. 2017;21:23–36. doi: 10.1080/14728222.2017.1265105. [DOI] [PubMed] [Google Scholar]
- 122.Carson C.F., Hammer K.A., Riley T.V. Melaleuca alternifolia (Tea Tree) oil: A review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev. 2006;19:50–62. doi: 10.1128/CMR.19.1.50-62.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rad J.S., Salehi B., Varoni E.M., Sharopov F., Yousaf Z., Ayatollahi S.A., Kobarfard F., Rad M.S., Afdjei M.H., Iriti M. Plants of the melaleuca genus as antimicrobial agents: From farm to pharmacy. Phytother. Res. 2017;31:1475–1494. doi: 10.1002/ptr.5880. [DOI] [PubMed] [Google Scholar]
- 124.Li X.Z., Plesiat P., Nikaido H. The challenge of efflux-mediated antibiotic resistance in gram-negative bacteria. Clin. Microbiol. Rev. 2015;28:337–418. doi: 10.1128/CMR.00117-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ni W., Li Y., Guan J., Zhao J., Cui J., Wang R., Liu Y. Effects of efflux pump inhibitors on colistin resistance in multidrug-resistant gram-negative bacteria. Antimicrob. Agents Chemother. 2016;60:3215–3218. doi: 10.1128/AAC.00248-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Selim S., Alfy S., Ruwaili M.A., Abdo A., Jaouni S.A. Susceptibility of imipenem-resistant Pseudomonas aeruginosa to flavonoid glycosides of date palm (Phoenix dactylifera L.) tamar growing in Al madinah, saudi arabia. Afr. J. Biotechnol. 2012;11:416–422. doi: 10.5897/AJB11.1412. [DOI] [Google Scholar]
- 127.Cowan M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999;12:564–582. doi: 10.1128/CMR.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Eumkeb G., Sakdarat S., Siriwong S. Reversing beta-lactam antibiotic resistance of Staphylococcus aureus with galangin from Alpinia officinarum hance and synergism with ceftazidime. Phytomedicine. 2010;18:40–45. doi: 10.1016/j.phymed.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 129.Eumkeb G., Siriwong S., Phitaktim S., Rojtinnakorn N., Sakdarat S. Synergistic activity and mode of action of flavonoids isolated from smaller galangal and amoxicillin combinations against amoxicillin-resistant Escherichia coli. J. Appl. Microbiol. 2012;112:55–64. doi: 10.1111/j.1365-2672.2011.05190.x. [DOI] [PubMed] [Google Scholar]
- 130.Davidson P.M., Naidu A.S. Phyto-phenols. In: Naidu A.S., editor. Natural Food Antimicrobial Systems. CRC Press; Boca Raton, FL, USA: 2000. pp. 278–307. [Google Scholar]
- 131.Burt S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 132.Gill A.O., Holley R.A. Mechanisms of bactericidal action of cinnamaldehyde against Listeria monocytogenes and of eugenol against L. monocytogenes and Lactobacillus sakei. Appl. Environ. Microbiol. 2004;70:5750–5755. doi: 10.1128/AEM.70.10.5750-5755.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gill A.O., Holley R.A. Disruption of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant oil aromatics. Int. J. Food Microbiol. 2006;108:1–9. doi: 10.1016/j.ijfoodmicro.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 134.Gill A.O., Holley R.A. Inhibition of membrane bound ATPases of Escherichia coli and Listeria monocytogenes by plant oil aromatics. Int. J. Food Microbiol. 2006;111:170–174. doi: 10.1016/j.ijfoodmicro.2006.04.046. [DOI] [PubMed] [Google Scholar]
- 135.Negi P.S. Plant extracts for the control of bacterial growth: Efficacy, stability and safety issues for food application. Int. J. Food Microbiol. 2012;156:7–17. doi: 10.1016/j.ijfoodmicro.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 136.Ozfenerci M., Calıskan U.K. Tea tree oil and its use in aromatherapy. Curr. Pers. Maps. 2018;2:90–102. [Google Scholar]
- 137.Lorenzi V., Muselli A., Bernardini A.F., Berti L., Pages J.M., Amaral L., Bolla J.M. Geraniol restores antibiotic activities against multidrug-resistant isolates from gram-negative species. Antimicrob. Agents Chemother. 2009;53:2209–2211. doi: 10.1128/AAC.00919-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Aqil F., Khan M.S., Owais M., Ahmad I. Effect of certain bioactive plant extracts on clinical isolates of beta-lactamase producing methicillin resistant Staphylococcus aureus. J. Basic. Microbiol. 2005;45:106–114. doi: 10.1002/jobm.200410355. [DOI] [PubMed] [Google Scholar]
- 139.Kondo K., Takaishi Y., Shibata H., Higuti T. ILSMRs (intensifier of beta-lactam-susceptibility in methicillin-resistant Staphylococcus aureus) from tara [caesalpinia spinosa (Molina) kuntze] Phytomedicine. 2006;13:209–212. doi: 10.1016/j.phymed.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 140.Rodrigues F.F., Costa J.G., Coutinho H.D. Synergy effects of the antibiotics gentamicin and the essential oil of croton zehntneri. Phytomedicine. 2009;16:1052–1055. doi: 10.1016/j.phymed.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 141.Grande M.J., Lopez R.L., Abriouel H., Valdivia E., Omar N.B., Maqueda M., Canamero M.M., Galvez A. Treatment of vegetable sauces with enterocin AS-48 alone or in combination with phenolic compounds to inhibit proliferation of Staphylococcus aureus. J. Food Prot. 2007;70:405–411. doi: 10.4315/0362-028X-70.2.405. [DOI] [PubMed] [Google Scholar]
- 142.Chan B.C., Ip M., Lau C.B., Lui S.L., Jolivalt C., Elbaz C.G., Litaudon M., Reiner N.E., Gong H., See R.H., et al. Synergistic effects of baicalein with ciprofloxacin against NorA over-expressed methicillin-resistant Staphylococcus aureus (MRSA) and inhibition of MRSA pyruvate kinase. J. Ethnopharmacol. 2011;137:767–773. doi: 10.1016/j.jep.2011.06.039. [DOI] [PubMed] [Google Scholar]
- 143.Nikaido H. Porins and specific diffusion channels in bacterial outer membranes. J. Biol. Chem. 1994;269:3905–3908. [PubMed] [Google Scholar]
- 144.Pieboji J.G., Baurin S., Frere J.M., Ngassam P., Ngameni B., Azebaze A., Pegnyemb D.E., Watchueng J., Goffin C., Galleni M. Screening of some medicinal plants from cameroon for beta-lactamase inhibitory activity. Phytother. Res. 2007;21:284–287. doi: 10.1002/ptr.2001. [DOI] [PubMed] [Google Scholar]
- 145.Zhao W.H., Hu Z.Q., Hara Y., Shimamura T. Inhibition of penicillinase by epigallocatechin gallate resulting in restoration of antibacterial activity of penicillin against penicillinase-producing Staphylococcus aureus. Antimicrob. Agents Chemother. 2002;46:2266–2268. doi: 10.1128/AAC.46.7.2266-2268.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Johny A.K., Hoagland T., Venkitanarayanan K. Effect of subinhibitory concentrations of plant-derived molecules in increasing the sensitivity of multidrug-resistant Salmonella enterica serovar typhimurium DT104 to antibiotics. Foodborne Pathog. Dis. 2010;7:1165–1170. doi: 10.1089/fpd.2009.0527. [DOI] [PubMed] [Google Scholar]
- 147.Moussaoui F., Alaoui T. Evaluation of antibacterial activity and synergistic effect between antibiotic and the essential oils of some medicinal plants. Asian Pac. J. Trop. Biomed. 2016;6:32–37. doi: 10.1016/j.apjtb.2015.09.024. [DOI] [Google Scholar]
- 148.Senatore F., Rigano D., Fusco R.D., Bruno M. Composition of the essential oil from flowerheads of Chrysanthemum coronarium L. (Asteraceae) growing wild in southern italy. Flavour. Fragr. 2004;19:149–152. doi: 10.1002/ffj.1285. [DOI] [Google Scholar]
- 149.Davey M.E., O’Toole G.A. Microbial biofilms: From ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 2000;64:847–867. doi: 10.1128/MMBR.64.4.847-867.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chmielewski R.A.N., Frank J.F. Biofilm formation and control in food processing facilities. Compr. Rev. Food Sci. Food Saf. 2003;2:22–32. doi: 10.1111/j.1541-4337.2003.tb00012.x. [DOI] [PubMed] [Google Scholar]
- 151.Flemming H.C., Wingender J. The biofilm matrix. Nat. Rev. Microbiol. 2010;8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 152.Langsrud S., Sidhu M.S., Heir E., Holck A.L. Bacterial disinfectant resistance—A challenge for the food industry. Int. Biodeterior. Biodegrad. 2003;51:283–290. doi: 10.1016/S0964-8305(03)00039-8. [DOI] [Google Scholar]
- 153.Simoes M., Bennett R.N., Rosa E.A. Understanding antimicrobial activities of phytochemicals against multidrug resistant bacteria and biofilms. Nat. Prod. Rep. 2009;26:746–757. doi: 10.1039/b821648g. [DOI] [PubMed] [Google Scholar]
- 154.Simões M., Simões L.C., Machado I., Pereira M.O., Vieira M.J. Control of flow-generated biofilms with surfactants: Evidence of resistance and recovery. Food Bioprod. Proces. 2006;84:338–345. doi: 10.1205/fbp06022. [DOI] [Google Scholar]
- 155.Upadhyay A., Upadhyaya I., Johny A.K., Venkitanarayanan K. Antibiofilm effect of plant derived antimicrobials on Listeria monocytogenes. Food Microbiol. 2013;36:79–89. doi: 10.1016/j.fm.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 156.Nostro A., Marino A., Blanco A.R., Cellini L., Di Giulio M., Pizzimenti F., Roccaro A.S., Bisignano G. In vitro activity of carvacrol against staphylococcal preformed biofilm by liquid and vapour contact. J. Med. Microbiol. 2009;58:791–797. doi: 10.1099/jmm.0.009274-0. [DOI] [PubMed] [Google Scholar]
- 157.Nostro A., Scaffaro R., D’Arrigo M., Botta L., Filocamo A., Marino A., Bisignano G. Study on carvacrol and cinnamaldehyde polymeric films: Mechanical properties, release kinetics and antibacterial and antibiofilm activities. Appl. Microbiol. Biotechnol. 2012;96:1029–1038. doi: 10.1007/s00253-012-4091-3. [DOI] [PubMed] [Google Scholar]
- 158.Nostro A., Sudano Roccaro A., Bisignano G., Marino A., Cannatelli M.A., Pizzimenti F.C., Cioni P.L., Procopio F., Blanco A.R. Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Med. Microbiol. 2007;56:519–523. doi: 10.1099/jmm.0.46804-0. [DOI] [PubMed] [Google Scholar]
- 159.Walencka E., Rozalska S., Wysokinska H., Rozalski M., Kuzma L., Rozalska B. Salvipisone and aethiopinone from salvia sclarea hairy roots modulate staphylococcal antibiotic resistance and express anti-biofilm activity. Planta Med. 2007;73:545–551. doi: 10.1055/s-2007-967179. [DOI] [PubMed] [Google Scholar]
- 160.Bakri A.G.A., Othman G., Afifi F.U. Determination of the antibiofilm, antiadhesive, and anti-MRSA activities of seven salvia species. Pharm. Mag. 2010;6:264–270. doi: 10.4103/0973-1296.71786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wu H., Lee B., Yang L., Wang H., Givskov M., Molin S., Hoiby N., Song Z. Effects of ginseng on Pseudomonas aeruginosa motility and biofilm formation. FEMS Immunol. Med. Microbiol. 2011;62:49–56. doi: 10.1111/j.1574-695X.2011.00787.x. [DOI] [PubMed] [Google Scholar]
- 162.Cheng K., Anderson D., Dan A. Inhibiting biofilm formation of Pseudomonas aeruginosa: A two-pronged attack. Young Sci. J. 2009;2:8–13. [Google Scholar]
- 163.Grudniak A.M., Kurek A., Szarlak J., Wolska K.I. Oleanolic and ursolic acids influence affect the expression of the cysteine regulon and the stress response in Escherichia coli. Curr. Microbiol. 2011;62:1331–1336. doi: 10.1007/s00284-010-9866-0. [DOI] [PubMed] [Google Scholar]
- 164.Ren D., Zuo R., Barrios A.F.G., Bedzyk L.A., Eldridge G.R., Pasmore M.E., Wood T.K. Differential gene expression for investigation of Escherichia coli biofilm inhibition by plant extract ursolic acid. Appl. Environ. Microbiol. 2005;71:4022–4034. doi: 10.1128/AEM.71.7.4022-4034.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Amalaradjou M.A., Narayanan A., Venkitanarayanan K. Trans-cinnamaldehyde decreases attachment and invasion of uropathogenic Escherichia coli in urinary tract epithelial cells by modulating virulence gene expression. J. Urol. 2011;185:1526–1531. doi: 10.1016/j.juro.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 166.Goh E.B., Yim G., Tsui W., McClure J., Surette M.G., Davies J. Transcriptional modulation of bacterial gene expression by subinhibitory concentrations of antibiotics. Proc. Natl. Acad. Sci USA. 2002;99:17025–17030. doi: 10.1073/pnas.252607699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Qiu J., Feng H., Lu J., Xiang H., Wang D., Dong J., Wang J., Wang X., Liu J., Deng X. Eugenol reduces the expression of virulence-related exoproteins in Staphylococcus aureus. Appl. Environ. Microbiol. 2010;76:5846–5851. doi: 10.1128/AEM.00704-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tsui W.H., Yim G., Wang H.H., McClure J.E., Surette M.G., Davies J. Dual effects of MLS antibiotics: Transcriptional modulation and interactions on the ribosome. Chem. Biol. 2004;11:1307–1316. doi: 10.1016/j.chembiol.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 169.Upadhyay A., Johny A.K., Amalaradjou M.A., Baskaran S.A., Kim K.S., Venkitanarayanan K. Plant-derived antimicrobials reduce Listeria monocytogenes virulence factors in vitro, and down-regulate expression of virulence genes. Int. J. Food Microbiol. 2012;157:88–94. doi: 10.1016/j.ijfoodmicro.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 170.Yim G., McClure J., Surette M.G., Davies J.E. Modulation of salmonella gene expression by subinhibitory concentrations of quinolones. J. Antibiot. 2011;64:73–78. doi: 10.1038/ja.2010.137. [DOI] [PubMed] [Google Scholar]
- 171.Brackman G., Defoirdt T., Miyamoto C., Bossier P., Calenbergh S.V., Nelis H., Coenye T. Cinnamaldehyde and cinnamaldehyde derivatives reduce virulence in vibrio spp. by decreasing the DNA-binding activity of the quorum sensing response regulator LuxR. BMC Microbiol. 2008;8:149. doi: 10.1186/1471-2180-8-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zou Y., Woo J., Ahn J. Cellular and molecular responses of salmonella typhimurium to antimicrobial-induced stresses during the planktonic-to-biofilm transition. Lett. Appl. Microbiol. 2012;55:274–282. doi: 10.1111/j.1472-765X.2012.03288.x. [DOI] [PubMed] [Google Scholar]
- 173.Jakobsen T.H., Gennip M.V., Phipps R.K., Shanmugham M.S., Christensen L.D., Alhede M., Skindersoe M.E., Rasmussen T.B., Friedrich K., Uthe F., et al. Ajoene, a sulfur-rich molecule from garlic, inhibits genes controlled by quorum sensing. Antimicrob. Agents Chemother. 2012;56:2314–2325. doi: 10.1128/AAC.05919-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Amalaradjou M.A., Narayanan A., Baskaran S.A., Venkitanarayanan K. Antibiofilm effect of trans-cinnamaldehyde on uropathogenic Escherichia coli. J. Urol. 2010;184:358–363. doi: 10.1016/j.juro.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 175.Dalleau S., Cateau E., Berges T., Berjeaud J.M., Imbert C. In vitro activity of terpenes against candida biofilms. Int. J. Antimicrob. Agents. 2008;31:572–576. doi: 10.1016/j.ijantimicag.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 176.Khan M.S., Ahmad I. Biofilm inhibition by cymbopogon citratus and syzygium aromaticum essential oils in the strains of Candida albicans. J. Ethnopharmacol. 2012;140:416–423. doi: 10.1016/j.jep.2012.01.045. [DOI] [PubMed] [Google Scholar]
- 177.Knowles J.R., Roller S., Murray D.B., Naidu A.S. Antimicrobial action of carvacrol at different stages of dual-species biofilm development by Staphylococcus aureus and Salmonella enterica serovar typhimurium. Appl. Environ. Microbiol. 2005;71:797–803. doi: 10.1128/AEM.71.2.797-803.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Antunes L.C., Ferreira R.B. Intercellular communication in bacteria. Crit. Rev. Microbiol. 2009;35:69–80. doi: 10.1080/10408410902733946. [DOI] [PubMed] [Google Scholar]
- 179.Novick R.P., Geisinger E. Quorum sensing in staphylococci. Annu. Rev. Genet. 2008;42:541–564. doi: 10.1146/annurev.genet.42.110807.091640. [DOI] [PubMed] [Google Scholar]
- 180.Quinn T., O’Mahony R., Baird A.W., Drudy D., Whyte P., Fanning S. Multi-drug resistance in Salmonella enterica: Efflux mechanisms and their relationships with the development of chromosomal resistance gene clusters. Curr. Drug Targets. 2006;7:849–860. doi: 10.2174/138945006777709548. [DOI] [PubMed] [Google Scholar]
- 181.Adonizio A.L., Downum K., Bennett B.C., Mathee K. Anti-quorum sensing activity of medicinal plants in southern florida. J. Ethnopharmacol. 2006;105:427–435. doi: 10.1016/j.jep.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 182.Bauer W.D., Mathesius U. Plant responses to bacterial quorum sensing signals. Curr. Opin. Plant Biol. 2004;7:429–433. doi: 10.1016/j.pbi.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 183.Teplitski M., Robinson J.B., Bauer W.D. Plants secrete substances that mimic bacterial N-acyl homoserine lactone signal activities and affect population density-dependent behaviors in associated bacteria. Mol. Plant. Microbe Interact. 2000;13:637–648. doi: 10.1094/MPMI.2000.13.6.637. [DOI] [PubMed] [Google Scholar]
- 184.Bodini S.F., Manfredini S., Epp M., Valentini S., Santori F. Quorum sensing inhibition activity of garlic extract and p-coumaric acid. Lett. Appl. Microbiol. 2009;49:551–555. doi: 10.1111/j.1472-765X.2009.02704.x. [DOI] [PubMed] [Google Scholar]
- 185.Cech N.B., Junio H.A., Ackermann L.W., Kavanaugh J.S., Horswill A.R. Quorum quenching and antimicrobial activity of goldenseal (Hydrastis canadensis) against methicillin-resistant Staphylococcus aureus (MRSA) Planta Med. 2012;78:1556–1561. doi: 10.1055/s-0032-1315042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Talekar S.J., Chochua S., Nelson K., Klugman K.P., Quave C.L., Vidal J.E. 220D-F2 from rubus ulmifolius kills Streptococcus pneumoniae planktonic cells and pneumococcal biofilms. PLoS ONE. 2014;9:e97314. doi: 10.1371/journal.pone.0097314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bazargani M.M., Rohloff J. Antibiofilm activity of essential oils and plant extracts against Staphylococcus aureus and Escherichia coli biofilms. Food Control. 2016;61:156–164. doi: 10.1016/j.foodcont.2015.09.036. [DOI] [Google Scholar]
- 188.Fisher K., Phillips C. The mechanism of action of a citrus oil blend against Enterococcus faecium and Enterococcus faecalis. J. Appl. Microbiol. 2009;106:1343–1349. doi: 10.1111/j.1365-2672.2008.04102.x. [DOI] [PubMed] [Google Scholar]
- 189.Huskins W.C., Huckabee C.M., O’Grady N.P., Murray P., Kopetskie H., Zimmer L., Walker M.E., Cochran R.L.S., Jernigan J.A., Samore M., et al. Intervention to reduce transmission of resistant bacteria in intensive care. N. Engl. J. Med. 2011;364:1407–1418. doi: 10.1056/NEJMoa1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mulyaningsih S., Youns M., Readi M.Z.E., Ashour M.L., Nibret E., Sporer F., Herrmann F., Reichling J., Wink M. Biological activity of the essential oil of Kadsura longipedunculata (Schisandraceae) and its major components. J. Pharm. Pharm. 2010;62:1037–1044. doi: 10.1111/j.2042-7158.2010.01119.x. [DOI] [PubMed] [Google Scholar]
- 191.Zomorodian K., Saharkhiz M.J., Rahimi M.J., Bandegi A., Shekarkhar G., Bandegani A., Pakshir K., Bazargani A. Chemical composition and antimicrobial activities of the essential oils from three ecotypes of Zataria multiflora. Pharm. Mag. 2011;7:53–59. doi: 10.4103/0973-1296.75902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hobby G.H., Quave C.L., Nelson K., Compadre C.M., Beenken K.E., Smeltzer M.S. Quercus cerris extracts limit Staphylococcus aureus biofilm formation. J. Ethnopharmacol. 2012;144:812–815. doi: 10.1016/j.jep.2012.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ranfaing J., Remy C.D., Lavigne J.P., Sotto A. Propolis potentiates the effect of cranberry (Vaccinium macrocarpon) in reducing the motility and the biofilm formation of uropathogenic Escherichia coli. PLoS ONE. 2018;13:e0202609. doi: 10.1371/journal.pone.0202609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hyams C., Camberlein E., Cohen J.M., Bax K., Brown J.S. The Streptococcus pneumoniae capsule inhibits complement activity and neutrophil phagocytosis by multiple mechanisms. Infect. Immun. 2010;78:704–715. doi: 10.1128/IAI.00881-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sanders M.E., Norcross E.W., Robertson Z.M., Moore Q.C., Fratkin J., Marquart M.E. The Streptococcus pneumoniae capsule is required for full virulence in pneumococcal endophthalmitis. Invest. Ophthalmol. Vis. Sci. 2011;52:865–872. doi: 10.1167/iovs.10-5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.O’Riordan K., Lee J.C. Staphylococcus aureus capsular polysaccharides. Clin. Microbiol. Rev. 2004;17:218–234. doi: 10.1128/CMR.17.1.218-234.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Potera C. Forging a link between biofilms and disease. Science. 1999;283:1837–1839. doi: 10.1126/science.283.5409.1837. [DOI] [PubMed] [Google Scholar]
- 198.Taylor C.M., Roberts I.S. Capsular polysaccharides and their role in virulence. Contrib. Microbiol. 2005;12:55–66. doi: 10.1159/000081689. [DOI] [PubMed] [Google Scholar]
- 199.Johny A.K., Mattson T., Baskaran S.A., Amalaradjou M.A., Babapoor S., March B., Valipe S., Darre M., Hoagland T., Schreiber D., et al. Reduction of salmonella enterica Serovar enteritidis colonization in 20-day-old broiler chickens by the plant-derived compounds trans-cinnamaldehyde and eugenol. Appl. Environ. Microbiol. 2012;78:2981–2987. doi: 10.1128/AEM.07643-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Derakhshan S., Sattari M., Bigdeli M. Effect of subinhibitory concentrations of cumin (Cuminum cyminum L.) seed essential oil and alcoholic extract on the morphology, capsule expression and urease activity of klebsiella pneumoniae. Int. J. Antimicrob. Agents. 2008;32:432–436. doi: 10.1016/j.ijantimicag.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 201.Shah J. The salicylic acid loop in plant defense. Curr. Opin. Plant. Biol. 2003;6:365–371. doi: 10.1016/S1369-5266(03)00058-X. [DOI] [PubMed] [Google Scholar]
- 202.Huang C.T., Stewart P.S. Reduction of polysaccharide production in Pseudomonas aeruginosa biofilms by bismuth dimercaprol (BisBAL) treatment. J. Antimicrob. Chemother. 1999;44:601–605. doi: 10.1093/jac/44.5.601. [DOI] [PubMed] [Google Scholar]
- 203.Mehreen A., Waheed M., Liaqat I., Arshad N. Phytochemical, antimicrobial, and toxicological evaluation of traditional herbs used to treat sore throat. Biomed. Res. Int. 2016;2016:1–9. doi: 10.1155/2016/8503426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Altemimi A., Lakhssassi N., Baharlouei A., Watson D.G., Lightfoot D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants. 2017;6:42. doi: 10.3390/plants6040042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Popova I.E., Hall C., Kubatova A. Determination of lignans in flaxseed using liquid chromatography with time-of-flight mass spectrometry. J. Chromatogr. A. 2009;1216:217–229. doi: 10.1016/j.chroma.2008.11.063. [DOI] [PubMed] [Google Scholar]
- 206.Stone M.R.L., Butler M.S., Phetsang W., Cooper M.A., Blaskovich M.A.T. Fluorescent antibiotics: New research tools to fight antibiotic resistance. Trends Biotechnol. 2018;36:523–536. doi: 10.1016/j.tibtech.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 207.Carradori S., Chimenti P., Fazzari M., Granese A., Angiolella L. Antimicrobial activity, synergism and inhibition of germ tube formation by crocus sativus-derived compounds against Candida spp. J. Enzym. Inhib. Med. Chem. 2016;31:189–193. doi: 10.1080/14756366.2016.1180596. [DOI] [PubMed] [Google Scholar]