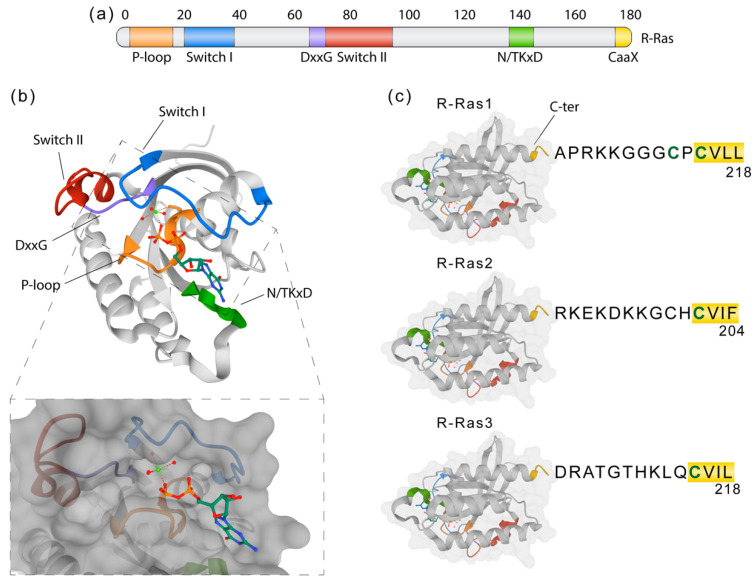
Figure 3.
R-Ras molecular structure and domains. (a) Representation of the R-Ras primary structure showing domains related to its function. Switch I and II (blue and red) are critical interfaces for downstream effectors and part of the nucleotide-binding pocket. The phosphate-binding loop (P-loop, orange) and the N/TKxD (green) regions are important for binding nucleotides, while the DxxG (violet) motif confers specificity for guanosine nucleotides. The CaaX C-terminal region is crucial for membrane attachment via prenylation or fatty acid modification. (b) Crystal structure of R-Ras1 bound to GDP (PDB: 2FN4) with highlighted regions corresponding to the ones in (a). GDP is shown as a ball and stick model, with each atom colored by element. The lower box represents the molecular surface model showing how GDP accommodates within the hydrophobic region. (c) The C-terminal ends of R-Ras1, R-Ras2, and R-Ras3, which constitute the “hypervariable region” (HVR), show significant sequence diversity important for their subcellular localization. CaaX box is underlined in yellow, with “C” being a cysteine substrate for prenylation, “a” any aliphatic amino acid, and “X” any amino acid.