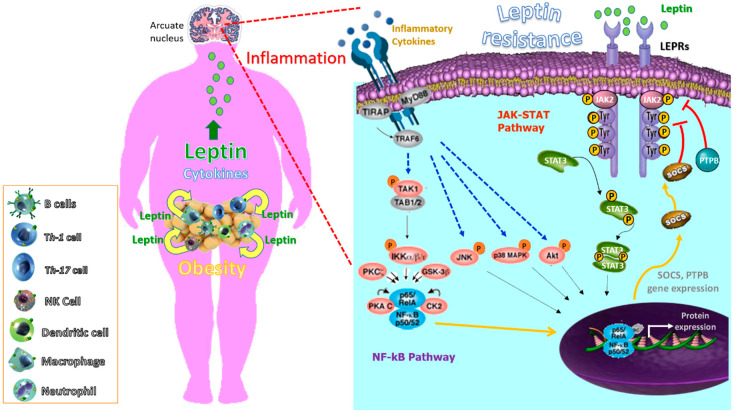
Figure 2.
Inflammation contributes to leptin resistance in the brain at the hypothalamic arcuate nucleus, and as a result, alters food intake and energy expenditure leading to obesity. Adipocytes and central nervous system (CNS) cells interact via several secreted factors. In obesity, the adipose tissue is characterized by hypertrophy and increased infiltration of macrophages and other immune cells. The metabolic consequences of adipose tissue dysfunction are an increased synthesis of proinflammatory cytokines and adipokines, such as leptin, which impair adipocyte function. This adipose tissue dysfunction leading to chronic inflammation, not only at the local level but also at the brain level. Moreover, inflammation produced by chronic infection and autoimmune diseases contribute to leptin resistance. Recruitment and activation of NF-KB signaling molecules by proinflammatory cytokines induce SOCS3 and protein tyrosine phosphatases-1B (PTP1B), which are involved in a negative feed-back loop to block LEPR signaling via the JAK/STAT pathway and promoting leptin resistance.