Abstract
Digoxin has been used for more than 50 years in patients with Atrial Fibrillation (AF), with the goal of Controlling Heart Rate (HR) and restoring sinus rhythm. In the last two decades, several studies have correlated therapeutic use of digoxin with increased mortality. However, such studies have potential biases that cannot be disregarded, mainly because they are cross-sectional experiments or post-hoc analyses of Randomized Controlled Trials (RCTs). Despite uncertainties regarding the safety of digoxin in this setting, it remains one of the most prescribed drugs for AF worldwide. On the other hand, the absence of any RCTs designed to evaluate mortality makes a definitive conclusion more difficult to reach; therefore, this medication must be used with care. In this review, we explored the therapeutic use of digoxin in the context of AF, discussed mortality data by means of critical analysis in the light of the best available evidence, and position ourselves in relation to more rigorous control of serum levels of this drug in daily practice.
Keywords: Digoxin, serum level, toxicity, mortality, heart failure, chronic atrial fibrillation
1. Introduction
Digoxin has been used for more than 200 years in the treatment of patients with Heart Failure (HF) [1]. In the early 20th century, James McKenzie and Thomas Lewis established the role of digitalis as the cornerstone of treatment for chronic Atrial Fibrillation (AF) [2], and for over 50 years it has played an important role as a therapeutic agent for heart-rate control in patients with this rhythm disturbance [3]. Over the years, digoxin has played a prominent role, but today it is viewed with suspicion by many cardiologists. European, American, and Canadian papers [4-6] reported increased adverse effects in patients with AF, and two meta-analyses [7, 8] found a possible association of digoxin with increased risk of all-cause mortality. Al-Zakwani et al. [9] found that the use of this drug in Middle Eastern patients with AF but without HF was associated with long-term mortality. On the other hand, other analyses have reported different results [10-12]. Recently, this topic has resurfaced due to the publication of a sub-analysis of the ARISTOTLE study, designed to compare apixaban vs. warfarin; this study found that patients with AF who presented serum digoxin levels ≥ 1.2 ng/ml were at higher risk of mortality [13].
Within this context, the present review discusses in detail the available data on the relationship between digoxin therapy and mortality in patients with AF. In addition, we will describe the mechanisms of action and pharmacology of digoxin, its main indications, and possible pathways that lead to greater risks of adverse effects.
2. Pharmacology and Mechanisms of Action
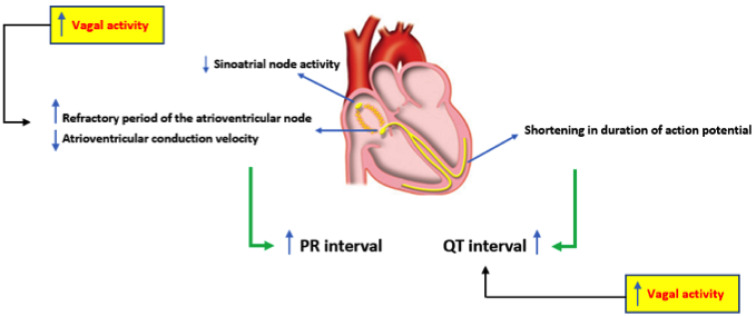
Digoxin works at different levels in the heart cells. It has positive inotropic effects and negative chronotropic and dromotropic action [14]. It also increases the availability of calcium to the contractile elements or myofibrils, inhibiting the sodium-potassium pump (Na+/K+-ATPase). As a consequence, the intracellular Na+ concentration increases, facilitating the entry of Ca2+ into the cell, which increases cardiac inotropism [15, 16]. Thus, the cardiac action potential will shorten, promoting negative chronotropism and positive inotropism due to increased calcium for the sarcomeric excitation-contraction coupling [1]. Digoxin also increases vagal activity, thus activating the vagal efferent nerves in the heart, a mechanism known as a parasympathomimetic effect [17]. It is believed that some important pathways by which digoxin increases the vagal tone are the improvement of the ganglionic transmission, sensitization of afferent systems, improvement in the ganglionic transmission in the efferent vagal nerves and increased organ responses peripheral to acetylcholine, a neurotransmitter that promotes heart rate reduction. With the increase of the vagal tone, the release of norepinephrine from the sympathetic terminals is expected to be inhibited by the interaction between the sympathetic and parasympathetic nervous systems [18]. Its action on sinus automaticity promotes heart rate reduction and lengthens atrioventricular conduction by reduction of the conduction velocity of electrical impulses through the atrioventricular node [19]. Thus, the prevailing concept of AF mechanism focuses on the interdependence of triggers and substrate, and monophasic atrial potentials recordings illustrate differences between normal myocardial properties and AF substrate, also for new-onset episodes. The main electrophysiological and electrocardiographic effects of digoxin are summarized in Fig. (1).
Fig. (1).
Electrophysiological and electrocardiographic effects of digoxin. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
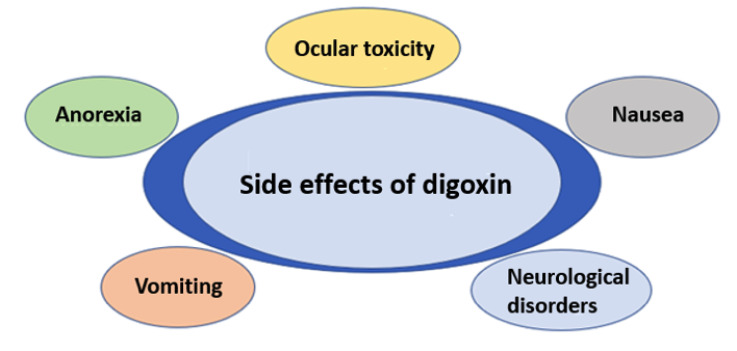
Digoxin is excreted mainly through glomerular filtration, but both the renal tubules and the liver also assist in this task [16]. The onset of action occurs 30 minutes to 2 hours after oral ingestion and 15 to 30 minutes after intravenous administration [20]. About 80% of an oral digoxin dose is absorbed, especially in the proximal small intestine [21]. Intramuscular use is not recommended since creatinine phosphokinase levels can increase considerably [20, 22] and this route has great pharmacokinetic variability. It should be noted that the dosage in obese individuals should be based on lean body mass since the distribution of the drug into adipose tissue is minimal [20]. In patients with normal renal function, the half-life is approximately 36 hours; this may be prolonged in those with renal insufficiency [23]. Like any other drug, digoxin may cause cardiac and systemic side effects [24-26], and various factors that predispose to digoxin toxicity should be taken into account [2, 23]. Some of the possible side effects with the use of digoxin are summarized in Fig. (2).
Fig. (2).
Possible side effects of digoxin use. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3. Therapeutic use and mortality data
In 1997, the indication for the therapeutic use of digoxin in patients with HF and normal sinus rhythm was tested in the DIG (Digitalis Investigation Group) study [27]. In this large Randomized Clinical Trial (RCT), more than 6,500 symptomatic individuals with ejection fraction < 45% were divided into two groups (digoxin and placebo). The results showed no difference in total mortality after almost 40 months of follow-up, but there was a significant reduction in hospitalizations and cardiac mortality due to HF in the digoxin group. However, more than 20 years have passed since the publication of the DIG study, and conflicting data regarding the safety of digoxin, particularly for patients with AF, are still being discussed. In a scenario that is poor in well-designed evidence (i.e. from RCTs), some observational studies have shown contrasting results [28, 29]. For example, a recent analysis of AF patients, including individuals with and without HF, found no association between digoxin treatment and mortality, even after adjustment for confounding factors with different models and propensity scores [29].
In contrast, in a retrospective analysis of a large cohort (n = 122,465, with 28,679 of these on digoxin), Turakhia et al. [28] found an increased risk of mortality in digoxin users after multivariate adjustment (95 vs. 67 per 1,000 people-years; p<0.001). This was consistent across all subgroups and was independent of drug adherence, renal dysfunction, HF, or concomitant therapy with beta-blockers or amiodarone. In a sub-analysis of the AFFIRM study [30], which included more than 4,000 patients with AF who were at high risk for stroke, digoxin was also associated with an expressive increase in mortality, even after controlling for comorbidities and propensity scores, regardless of gender and the presence or absence of underlying HF. All-cause mortality was 41% higher in HF-AF patients vs. 37% in those without HF. Two meta-analyses including more than 300,000 patients each also found increased mortality with the use of digoxin in patients with AF, regardless of the presence of HF [7, 31].
In a large observational study [13] of patients with AF, the authors analyzed prevalence (those who were already on the drug at the start of the study) and incidence (use was initiated at some point during the study period) of digoxin use. The researchers compared these individuals using clinical variables (characteristics of AF, clinical history, concomitant medications) and laboratory parameters (NT-proBNP, troponin, and GDF-15), and markers were considered quite sensitive and correlated with mortality under these circumstances. After a very sophisticated analysis, they showed a significant dose-response curve between serum digoxin levels and mortality: for each 0.5-ng/mL increase in the former, there was a 19% increase in mortality. In addition, initiation of digoxin treatment during the study was associated with an increased risk of sudden cardiac death compared to participants in the control group, regardless of the state of HF. In these new users, the risk of sudden death was four times greater, especially in the first 6 months of use [13]. Although the authors have not studied the possible causes for this outcome, the toxicity of digoxin is well-known and may lead to malignant arrhythmias that increase mortality in these patients [32]. Besides that, initiating digoxin in patients with AF was significantly associated with an increased risk of HF hospitalization, primarily among patients with previous HF. In this scenario and for patient safety, target serum concentrations should be 0.5-0.9 ng/mL, which is in the lower part of the so-called ‘therapeutic’ range.
In the last year, two meta-analyses were published with contrasting results. Sethy et al. [33] observed possible selection and prescribing biases as well as differences in follow-up time and, therefore, could not confirm or reject the findings of increased mortality with the use of digoxin reported by previous reviews [33].
On the other hand, the most up-to-date meta-analysis on the subject grouped approximately 40 studies and more than 820,000 patients. Digoxin use was associated with a 17% increase in the relative risk of all-cause mortality (hazard ratio: 1.17, p<0.01). In the subgroup of patients with AF (over 600,000), digoxin treatment was also associated with a significant risk of death (hazard ratio: 1.23, p<0.01). Finally, the same occurred in the subgroup of approximately 200,000 patients with HF (hazard ratio: 1.11, p<0.01). When analyzing data from studies with new users of digoxin (i.e., incident users; more than 40,000 patients), a very high risk of death from all causes was demonstrated (hazard ratio: 1.47, p<0.01). The findings of this robust meta-analysis reinforce the importance of care when prescribing digoxin in different settings [34].
However, despite all this debate and the controversies surrounding it, digoxin is still commonly prescribed and used. Although it is no longer considered a first-line agent for heart rate control in AF [35], it is still an attractive option, since it has no negative inotropic effects [36]. Its use has decreased considerably in the last 30 years [37], but it is assumed that approximately 30% of patients with AF are still treated with this agent worldwide [29, 38]. When reading the available literature, the clinical cardiologist should take into account that all analyses that found an increased risk of adverse outcomes with the use of digoxin were based on observational studies or post-hoc analyses of RCTs, which may limit the quality of the evidence.
4. Measurement of serum digoxin level and relevance of its monitoring
A meta-analysis that included 19 major studies evaluated the association between digoxin use and mortality [31]. In only three of these studies were serum digoxin levels measured: one in AF [32] and two in HF [27, 39]. It bears noting that, as early as 1997, the DIG study showed that serum concentrations equal to or greater than 1.2 ng/ml were associated with a 56% increase in the risk of death when compared to patients who were not taking the drug [13]. A post-hoc analysis of the same DIG study, published more than a decade ago, showed that digoxin treatment was safe at serum concentrations between 0.5 and 0.9 ng/ml when compared at a serum level >1.0 ng/mL [39].
Following this reasoning, an interesting study was carried out through the measurement of serum digoxin and evaluation of platelet activation. Through a post-hoc analysis of a prospective study of patients with AF using anticoagulants, serum digoxin was associated with increased urinary excretion of thromboxane B2. In addition, in vitro experiments showed that digoxin increased platelet activation in pre-stimulated platelets, but patients with serum digoxin levels <1 ng/mL had no difference in platelet activation when compared to those who did not use the drug. However, the authors observed an increase in cardiovascular risk when serum levels were >1.2 ng/mL [40]. These findings corroborate the data described above [39], indicating the importance of maintaining digoxin levels below 1 ng/mL [40].
In another setting, including patients with end-stage renal disease on hemodialysis, there was an increased risk of overall mortality with concomitant digoxin therapy. Again, the safest serum levels were <0.9 ng/ml [41]. In this regard, there is at least one report in the literature describing that hemodialysis patients who had their digoxin dose increased from 0.125 to 0.25 mg three times a week had a toxic blood level of 4.9 ng/ml [42]. Finally, in 2010, in response to this evidence, the Heart Failure Society of America stated in its guideline that serum digoxin concentrations should be < 1.0 ng/ml, preferably from 0.7 to 0.9 ng/ml [43].
Although current U.S. [44] and European guidelines [45] do not specifically recommend measuring serum digoxin levels in patients with AF, it will not come as a surprise to us if they include such procedure in their next updates. As an aside, the main current indications for the therapeutic use of digoxin by different societies [35, 44-46] are detailed in Table 1.
Table 1.
Recommendations for the use of digoxin in the clinical management of atrial fibrillation by different societies.
| II Brazilian Guidelines for Atrial Fibrillation [ 35 ] |
|---|
| • May be combined with BB or CCB for better control of ventricular response - Class IIa, evidence level B |
| • Use in the presence of ventricular pre-excitation and AF - Class III, level of evidence B |
| Brazilian Guideline for Chronic and Acute Heart Failure [46] |
| • For symptomatic LV dysfunction despite optimal therapy with triple therapy to reduce symptoms and hospitalizations - Class IIa, evidence level B |
| • For LV dysfunction in patients with symptomatic AF despite optimized therapy (including BB) for ventricular rate control - Class IIa, evidence level B |
| • For asymptomatic LV dysfunction or with HFpEF in sinus rhythm - Class III, evidence level C |
| • To control HR in patients with symptomatic AF despite of optimized therapy (including BB or when this is not tolerated or contraindicated) - Class IIa, evidence level B |
| • For asymptomatic LV dysfunction or HFpEF in sinus rhythm - Class III |
| 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation [44] |
| • In patients with pre-excitation and AF, should not be administered - Class III, evidence level B • It may be considered as a retarder of the rapid ventricular response with acute coronary syndrome and AF and severe LV and HF or hemodynamic instability - Class IIb, evidence level C |
| 2016 ESC Guidelines for the management of atrial fibrillation [45] |
| • Recommended for HR control in patients with AF and LVEF < 40% - Class I, evidence level B • Recommended for HR control in patients with AF and LVEF ≥ 40% - Class I, evidence level B |
Abbreviations: AF: Atrial Fibrillation; BB: Beta-blockers; CCB: Calcium Channel Blockers; HF: Heart Failure; HFpEF: Heart Failure with Preserved Ejection Fraction; HR: Heart Rate; IV: Intravenous; LV: Left Ventricular; LVEF: Left Ventricular Ejection Fraction.
Conclusion
Despite the debate and controversy regarding the safety of digoxin therapy in patients with AF, it is still prescribed on a large scale. It is important to emphasize that, in a patient with AF whose heart rate cannot be controlled with other drugs, digoxin may be considered. It is essential for doctors to use the appropriate scientific evidence and to fully analyze the risk-effectiveness balance. When used chronically, low doses should always be preferred, and checking digoxin serum levels from time to time is advised.
Acknowledgements
Declared none.
Authors’ Contributions
Study design: Ferrari F; Data collection: Ferrari F, Stein R; Analysis and interpretation of data, manuscript writing: Ferrari F, Santander IRMF, Stein R; Critical review of the manuscript for important intellectual content: Stein R.
Consent for Publication
Not applicable.
Funding
Hospital de Clínicas de Porto Alegre Research and Event Incentive Fund (FIPE-HCPA), Brazilian Research Council (CNPq).
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Virgadamo S., Charnigo R., Darrat Y., Morales G., Elayi C.S. Digoxin: A systematic review in atrial fibrillation, congestive heart failure and post myocardial infarction. World J. Cardiol. 2015;7(11):808–816. doi: 10.4330/wjc.v7.i11.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tamargo J., Delpón E., Caballero R. The safety of digoxin as a pharmacological treatment of atrial fibrillation. Expert Opin. Drug Saf. 2006;5(3):453–467. doi: 10.1517/14740338.5.3.453. [DOI] [PubMed] [Google Scholar]
- 3.Scalese M.J., Salvatore D.J. Role of digoxin in atrial fibrillation. J. Pharm. Pract. 2017;30(4):434–440. doi: 10.1177/0897190016642361. [DOI] [PubMed] [Google Scholar]
- 4.Hallberg P., Lindbäck J., Lindahl B., Stenestrand U., Melhus H. Digoxin and mortality in atrial fibrillation: A prospective cohort study. Eur. J. Clin. Pharmacol. 2007;63(10):959–971. doi: 10.1007/s00228-007-0346-9. [DOI] [PubMed] [Google Scholar]
- 5.Pastori D., Farcomeni A., Bucci T., et al. Digoxin treatment is associated with increased total and cardiovascular mortality in anticoagulated patients with atrial fibrillation. Int. J. Cardiol. 2015;180:1–5. doi: 10.1016/j.ijcard.2014.11.112. [DOI] [PubMed] [Google Scholar]
- 6.Chao T.F., Liu C.J., Chen S.J., et al. Does digoxin increase the risk of ischemic stroke and mortality in atrial fibrillation? A nationwide population-based cohort study. Can. J. Cardiol. 2014;30(10):1190–1195. doi: 10.1016/j.cjca.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z.Q., Zhang R., Chen M.T., et al. Digoxin Is associated with increased all-cause mortality in patients with atrial fibrillation regardless of concomitant heart failure: A meta-analysis. J. Cardiovasc. Pharmacol. 2015;66(3):270–275. doi: 10.1097/FJC.0000000000000274. [DOI] [PubMed] [Google Scholar]
- 8.Qureshi W., O’Neal W.T., Soliman E.Z., Al-Mallah M.H. Systematic review and meta-analysis of mortality and digoxin use in atrial fibrillation. Cardiol. J. 2016;23(3):333–343. doi: 10.5603/CJ.a2016.0016. [DOI] [PubMed] [Google Scholar]
- 9.Al-Zakwani I., Panduranga P., Zubaid M., et al. Impact of digoxin on mortality in patients with atrial fibrillation stratified by heart failure: Findings from gulf survey of atrial fibrillation events in the middle east. J. Cardiovasc. Pharmacol. Ther. 2016;21(3):273–279. doi: 10.1177/1074248415603505. [DOI] [PubMed] [Google Scholar]
- 10.Gheorghiade M., Fonarow G.C., van Veldhuisen D.J., et al. Lack of evidence of increased mortality among patients with atrial fibrillation taking digoxin: Findings from post hoc propensity-matched analysis of the AFFIRM trial. Eur. Heart J. 2013;34(20):1489–1497. doi: 10.1093/eurheartj/eht120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friberg L., Hammar N., Rosenqvist M. Digoxin in atrial fibrillation: Report from the stockholm cohort study of atrial fibrillation (SCAF). Heart. 2010;96(4):275–280. doi: 10.1136/hrt.2009.175786. [DOI] [PubMed] [Google Scholar]
- 12.Rodríguez-Mañero M., Otero-Raviña F., García-Seara J., et al. Outcomes of a contemporary sample of patients with atrial fibrillation taking digoxin: Results from the AFBAR study. Rev. Esp. Cardiol. (Engl. Ed.) 2014;67(11):890–897. doi: 10.1016/j.rec.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 13.Lopes R.D., Rordorf R., De Ferrari G.M., et al. Digoxin and mortality in patients with atrial fibrillation. J. Am. Coll. Cardiol. 2018;71(10):1063–1074. doi: 10.1016/j.jacc.2017.12.060. [DOI] [PubMed] [Google Scholar]
- 14.Currie G.M., Wheat J.M., Kiat H. Pharmacokinetic considerations for digoxin in older people. Open Cardiovasc. Med. J. 2011;5:130–135. doi: 10.2174/1874192401105010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauman J.L., Didomenico R.J., Galanter W.L. Mechanisms, manifestations, and management of digoxin toxicity in the modern era. Am. J. Cardiovasc. Drugs. 2006;6(2):77–86. doi: 10.2165/00129784-200606020-00002. [DOI] [PubMed] [Google Scholar]
- 16.Bhatia S.J., Smith T.W. Digitalis toxicity: Mechanisms, diagnosis, and management. J. Card. Surg. 1987;2(4):453–465. doi: 10.1111/j.1540-8191.1987.tb00204.x. [DOI] [PubMed] [Google Scholar]
- 17.Rebagay W.R., Caldwell R.W. Different patterns of autonomic nerve activity produced by a polar vs. a neutral cardiac glycoside. J. Pharmacol. Exp. Ther. 1990;253(1):180–184. [PubMed] [Google Scholar]
- 18.Watanabe A.M. Digitalis and the autonomic nervous system. J. Am. Coll. Cardiol. 1985;5(5) Suppl. A:35A–42A. doi: 10.1016/S0735-1097(85)80461-7. [DOI] [PubMed] [Google Scholar]
- 19.Cheng J.W., Rybak I. Use of digoxin for heart failure and atrial fibrillation in elderly patients. Am. J. Geriatr. Pharmacother. 2010;8(5):419–427. doi: 10.1016/j.amjopharm.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Ehle M., Patel C., Giugliano R.P. Digoxin: clinical highlights: A review of digoxin and its use in contemporary medicine. Crit. Pathw. Cardiol. 2011;10(2):93–98. doi: 10.1097/HPC.0b013e318221e7dd. [DOI] [PubMed] [Google Scholar]
- 21.Ziff O.J., Kotecha D. Digoxin: The good and the bad. Trends Cardiovasc. Med. 2016;26(7):585–595. doi: 10.1016/j.tcm.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Greenblatt D.J., Duhme D.W., Koch-Weser J. Pain and CPX elevation after intramuscular digoxin. N. Engl. J. Med. 1973;288(13):689. doi: 10.1056/NEJM197303292881317. [DOI] [PubMed] [Google Scholar]
- 23.MacLeod-Glover N., Mink M., Yarema M., Chuang R. Digoxin toxicity: Case for retiring its use in elderly patients? Can. Fam. Physician. 2016;62(3):223–228. [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng W.T., Liu Z.H., Li Z.Y., Zhang M., Cheng Y.J. Digoxin use and adverse outcomes in patients with atrial fibrillation. Medicine (Baltimore) 2016;95(12):e2949. doi: 10.1097/MD.0000000000002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Renard D., Rubli E., Voide N., Borruat F.X., Rothuizen L.E. Spectrum of digoxin-induced ocular toxicity: A case report and literature review. BMC Res. Notes. 2015;8:368. doi: 10.1186/s13104-015-1367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amjad W., Qureshi W., Farooq A., et al. Gastrointestinal side effects of antiarrhythmic medications: A review of current literature. Cureus. 2017;9(9):e1646. doi: 10.7759/cureus.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The effect of digoxin on mortality and morbidity in patients with heart failure. N. Engl. J. Med. 1997;336(8):525–533. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 28.Turakhia M.P., Santangeli P., Winkelmayer W.C., et al. Increased mortality associated with digoxin in contemporary patients with atrial fibrillation: Findings from the TREAT-AF study. J. Am. Coll. Cardiol. 2014;64(7):660–668. doi: 10.1016/j.jacc.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allen L.A., Fonarow G.C., Simon D.N., et al. Digoxin use and subsequent outcomes among patients in a contemporary atrial fibrillation cohort. J. Am. Coll. Cardiol. 2015;65(25):2691–2698. doi: 10.1016/j.jacc.2015.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whitbeck M.G., Charnigo R.J., Khairy P., et al. Increased mortality among patients taking digoxin--analysis from the AFFIRM study. Eur. Heart J. 2013;34(20):1481–1488. doi: 10.1093/eurheartj/ehs348. [DOI] [PubMed] [Google Scholar]
- 31.Vamos M., Erath J.W., Hohnloser S.H. Digoxin-associated mortality: A systematic review and meta-analysis of the literature. Eur. Heart J. 2015;36(28):1831–1838. doi: 10.1093/eurheartj/ehv143. [DOI] [PubMed] [Google Scholar]
- 32.Freeman J.V., Reynolds K., Fang M., et al. Digoxin and risk of death in adults with atrial fibrillation: The ATRIA-CVRN study. Circ Arrhythm Electrophysiol. 2015;8(1):49–58. doi: 10.1161/CIRCEP.114.002292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sethi N.J., Nielsen E.E., Safi S., Feinberg J., Gluud C., Jakobsen J.C. Digoxin for atrial fibrillation and atrial flutter: A systematic review with meta-analysis and trial sequential analysis of randomised clinical trials. PLoS One. 2018;13(3):e0193924. doi: 10.1371/journal.pone.0193924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vamos M., Erath J.W., Benz A.P., Lopes R.D., Hohnloser S.H. Meta-analysis of effects of digoxin on survival in patients with atrial fibrillation or heart failure: An update. Am. J. Cardiol. 2019;123(1):69–74. doi: 10.1016/j.amjcard.2018.09.036. [DOI] [PubMed] [Google Scholar]
- 35.Magalhães L.P., Figueiredo M.J.O., Cintra F.D., et al. II diretrizes brasileiras de fibrilação atrial. Arq. Bras. Cardiol. 2016;106(4) Suppl. 2:1–22. doi: 10.5935/abc.20160055. [DOI] [Google Scholar]
- 36.Tariq S., Aronow W.S. Use of inotropic agents in treatment of systolic heart failure. Int. J. Mol. Sci. 2015;16(12):29060–29068. doi: 10.3390/ijms161226147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldberger Z.D., Alexander G.C. Digitalis use in contemporary clinical practice: Refitting the foxglove. JAMA Intern. Med. 2014;174(1):151–154. doi: 10.1001/jamainternmed.2013.10432. [DOI] [PubMed] [Google Scholar]
- 38.Washam J.B., Stevens S.R., Lokhnygina Y., et al. Digoxin use in patients with atrial fibrillation and adverse cardiovascular outcomes: A retrospective analysis of the Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF). Lancet. 2015;385(9985):2363–2370. doi: 10.1016/S0140-6736(14)61836-5. [DOI] [PubMed] [Google Scholar]
- 39.Ahmed A., Gambassi G., Weaver M.T., Young J.B., Wehrmacher W.H., Rich M.W. Effects of discontinuation of digoxin vs. continuation at low serum digoxin concentrations in chronic heart failure. Am. J. Cardiol. 2007;100(2):280–284. doi: 10.1016/j.amjcard.2007.02.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pastori D., Carnevale R., Nocella C., et al. Digoxin and platelet activation in patients with atrial fibrillation: In vivo and in vitro study. J. Am. Heart Assoc. 2018;7(22):e009509. doi: 10.1161/JAHA.118.009509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan K.E., Lazarus J.M., Hakim R.M. Digoxin associates with mortality in ESRD. J. Am. Soc. Nephrol. 2010;21(9):1550–1559. doi: 10.1681/ASN.2009101047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Papadakis M.A., Wexman M.P., Fraser C., Sedlacek S.M. Hyperkalemia complicating digoxin toxicity in a patient with renal failure. Am. J. Kidney Dis. 1985;5(1):64–66. doi: 10.1016/S0272-6386(85)80139-6. [DOI] [PubMed] [Google Scholar]
- 43.Lindenfeld J., Albert N.M., Boehmer J.P., et al. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J. Card. Fail. 2010;16(6) doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 44.January C.T., Wann L.S., Alpert J.S., et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2014;64(21):e1–e76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 45.Kirchhof P., Benussi S., Kotecha D., et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016;37(38):2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 46.Rohde L.E.P., Montera M.W., Bocchi E.A., et al. Diretriz brasileira de insuficiência cardíaca crônica e aguda. Arq. Bras. Cardiol. 2018;111(3):436–539. doi: 10.5935/abc.20180190. [DOI] [PubMed] [Google Scholar]