Figure 1.
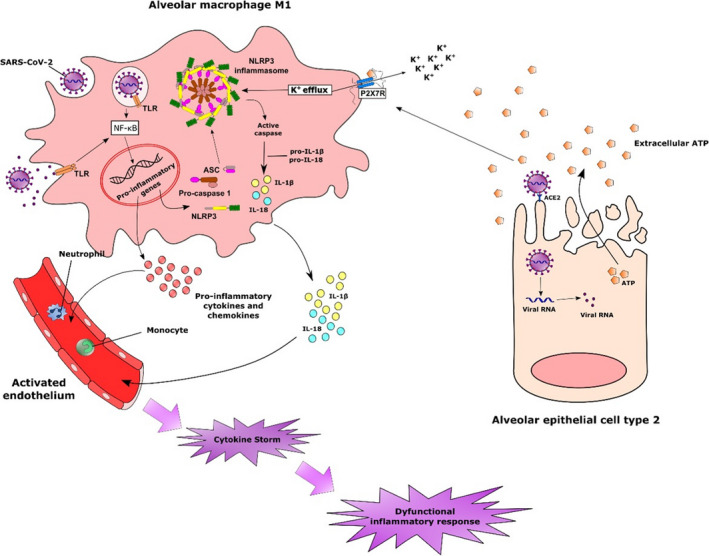
Possible involvement of alveolar macrophages in the inflammatory response triggered by SARS‐CoV‐2 infection. The rapid infection of susceptible cells can lead to sustained inflammatory response by the release of intracellular contents. The accumulation of extracellular ATP in concentrations sufficient to activate P2X7R associated with the recognition of viral products triggers several intracellular mechanisms that culminate with the expression of inflammatory genes, including those related to the NLRP3 inflammasome. The activation of P2X7R in alveolar macrophages with the consequent K+ efflux constitutes a potent signal for the assembly of NLRP3 inflammasome. The formation of this multiprotein complex leads to the activation of caspase‐1, which in turn cleaves pro‐IL‐1β and IL‐18 into their bioactive forms. Alternatively, active caspase‐1 can also cleave the protein gasdermin D, inducing pyroptosis. The release of IL1‐β and other cytokines and chemokines from activated macrophages results in the activation of the endothelium and infiltration of other inflammatory cells, such as monocytes and neutrophils, that will contribute to the amplification of the inflammatory response.
