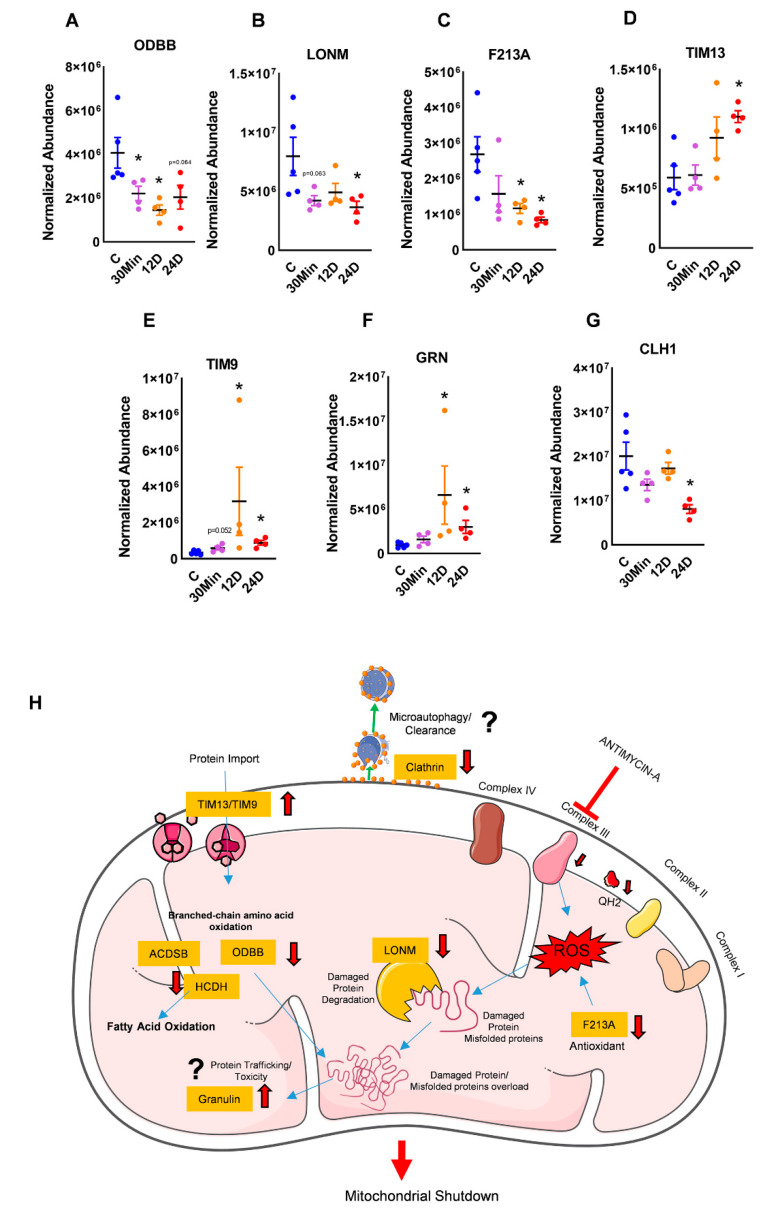
Figure 3.
Complex III inhibition instigated dysfunctional protein metabolism and folding with oxidative stress. (A) 2-Oxoisovalerate dehydrogenase subunit beta (ODBB), a protein that metabolizes BCCAs or branched-chain amino acids, was found to be significantly downregulated in complex III-inhibited groups. (B) Lon protease homolog (LONM), a protein that repairs damaged polypeptides and (C) peroxiredoxin-like 2A (PRXL2A or F213A), an antioxidant protein, were found to be decreased in complex III-inhibited groups. (D) TIM13 and (E) TIM9 peptide transport proteins were significantly upregulated with complex III inhibition. (F) Granulin (GRN), a trafficking protein, and (G) clathrin (CLH1), a protein assisting microautophagy, were significantly decreased with complex III inhibition. (H) Decreased amino acid metabolism in conjunction with decreased protein repair and oxidative damage clearance would cause a toxic mitochondrial overload. This, in conjunction with increased inflammation and reduced microautophagy, could potentially shut down mitochondrial function. Red ‘T’ arrow shows inhibition, red arrows show downregulation and all other arrows show transfer (mean ± SEM, n = 4-5, * vs. 0h treatment, p < 0.05, t-test).