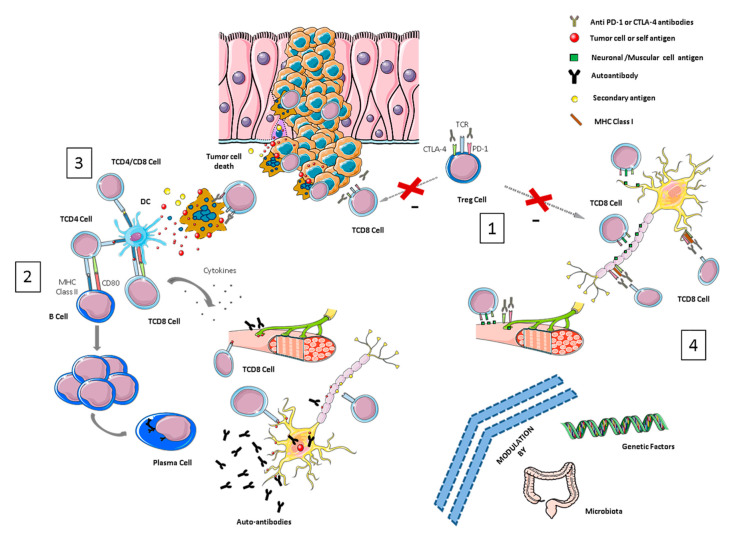
Figure 1.
Overview of pathological mechanisms underlying neurological immune-related toxicities (NirAEs). (1) Regulatory T-cells (Tregs) are key players in maintaining immune tolerance and tumor immune-suppression within the tumor microenvironment by suppressing effector T-cells. CTLA-4 and PD-1 are receptors expressed by these cells. Treg cell blockade by immune checkpoint inhibitors (ICIs) may induce Treg reduction and the disruption of CD8 + T-cell control, resulting in the proliferation and activation of effector cells. Thus, an immune toxicity development can be started through the recognition of self-tissue antigens, such as neuronal or muscular antigens. (2) The molecular similarity between a tumor-antigen and a self-antigen recognized by effector cells might induce T- or B-autoreactive cells wrongly directed against self-antigens in a process called molecular mimicry, inducing autoimmune reactions against normal tissues. (3) Cytotoxic CD8+ T-cells, after recognizing tumor antigens, induce cytokine secretion and the cytotoxic death of tumor cells. This process can also wrongly produce the death of non-transformed bystander cells releasing secondary antigens. The primary and secondary antigens released by both types of cells (antigen spreading) might be ingested and processed by antigen-presenting cells (APCs). These activated APCs can prime new T- or B-cells that can cause additional tumor and normal tissue destruction, leading to an autoimmunity process. (4) ICI antibodies could recognize their target molecules, which are also expressed by non-hematopoietic cells including those of the nervous system (such as astrocytes and neurons) and thereby directly induce local injury through antibodies or T-cell cytotoxicity mechanisms.