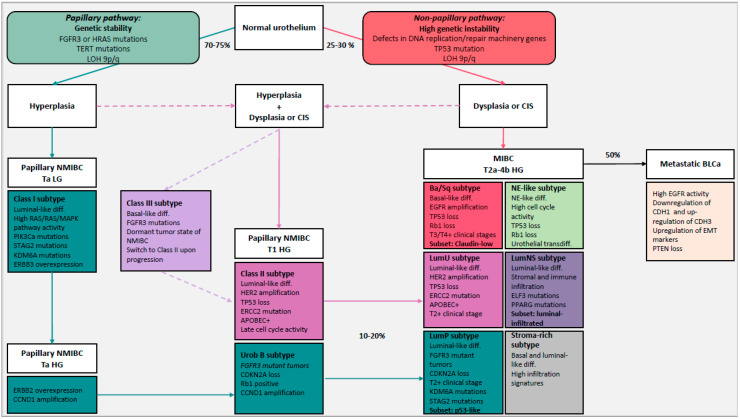
Figure 1.
Simplified representation of the evolution of bladder cancer (BLCa) including the two distinct carcinogenic pathways (papillary and non-papillary pathways) and molecular subtypes of BLCa with their different characteristics. 75–80% of BLCa are non-muscle invasive bladder cancer (NMIBC) and 20–25% are muscle invasive bladder cancer (MIBC), of which 50% progress to metastatic BLCa. The papillary pathway includes hyperplasia which will give rise to Ta low-grade (LG) NMIBC and Ta/T1 high-grade (HG) NMIBC after the acquisition of mutations such as fibroblast growth factor receptor 3 (FGFR3) mutations or RAS mutations, loss of heterozygosity (LOH) 9p/q and telomerase reverse transcriptase (TERT) gene-promoter mutations. Class I/Urobasal A (Uro A) subtypes represent Ta LG NMIBC tumors and Uro B tumors were defined to be their progressed version. 10–20% of NMIBC potentially progress to MIBC and given the elevated expression of FGFR3 and the homozygous cyclin dependent kinase inhibitor 2A (CDKN2A) deletions in luminal papillary (LumP) tumors, they can originate from the progression of Class I/Uro A tumors through the papillary pathway. In the non-papillary pathway, instead, flat dysplasia and/or carcinoma in situ (CIS) are believed to be the precursors of MIBC tumors, after the gradual accumulation of genomic abnormalities such as loss of tumor suppressors TP53, defects in DNA replication/repair machinery genes (e.g., excision repair 2 (ERCC2)) and LOH 9q/p. Basal/Squamous (Ba/Sq) subtype represents the fraction of MIBC that originate directly from the non-papillary pathway. Given their genomic instability, some HG NMIBC tumors seem to derive from the co-occurrence of hyperplasia and dysplasia shown by the dashed arrows. Class II subtypes represent T1 HG NMIBC tumors that originate from the co-occurrence of hyperplasia and dysplasia, and luminal unstable (LumU)/genomic unstable (GU) tumors are the progressed version that advanced through the non-papillary pathway. Luminal non-specified (LumNS) exhibits features of the LumP and LumU subtypes. Class III represents a dormant tumor state of NMIBC that seems to switch to Class II upon progression. However, the origin of Class III tumors is not fully clear. It is probably a fraction of HG tumors that originated from both papillary and non-papillary pathways given the basal phenotype and FGFR3 mutations. The neuroendocrine-like (NE-like) subtype expresses neuroendocrine differentiation markers and its origin is not fully clear. They maybe originate from transdifferentiation of urothelial cells upon treatment. The stroma-rich subtype, associated with high infiltration signatures, such as LumNS, represents a heterogeneous class of tumors that we need to investigate at a higher resolution. Blue arrow indicates reoccurrence of NMIBC. Dashed arrows are hypothetical. Abbreviation: PI3KCa: phosphatidylinositol-4,5-bisphosphate 3-binase catalytic subunit alpha, STAG2: stromal antigen 2, KDM6A: lysine demethylase 6A, ERBB2/3: Erb-B2 receptor tyrosine kinase 2/3, CCND1: cyclin D1, EGFR: epidermal growth factor receptor, HER2: human epidermal growth factor receptor 2, APOBEC: apoliprotein B mRNA-editing enzyme catalytic polypeptide-like, PPARG: peroxisome proliferator activated receptor gamma, ELF3: E74 Like ETS transcription factor 3, CDH1: cadherin 1, CDH3: cadherin 3, EMT: epithelial-to-mesenchymal transition, PTEN: phosphatase and tensin homolog, diff.: differentiation, inf: infiltration.