Scheme 2. General Stepwise Synthesis of Sequence-Defined Tricarb Macrocyclesa.
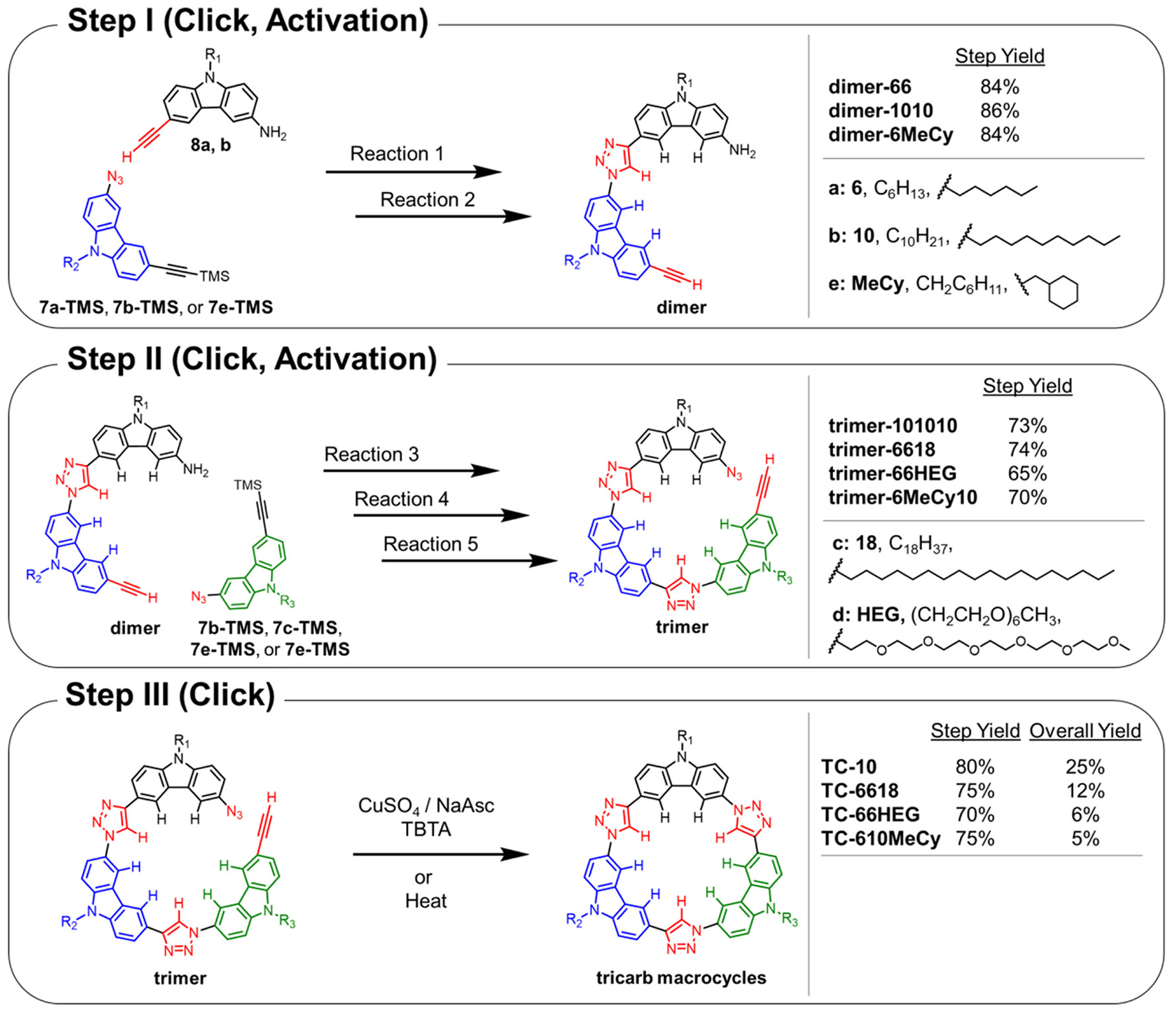
aStep I: TMS-protected carbazoles (7a-TMS, 7b-TMS, or 7e-TMS) are coupled with amine-substituted carbazoles (8a or 8b) and, after deprotection, give the corresponding crescent dimer. Step II: The crescent dimer is coupled with an additional TMS-protected carbazole building block (7b-TMS to 7d-TMS). Step III: Following installation of the azide and deprotection of the alkyne, the crescent trimer can be closed to produce the tricarb macrocycle. Reactions 1 and 3: CuSO4/NaAsc/TBTA/2:1:1 THF/EtOH/H2O/55 °C/Ar. Reactions 2 and 5: K2CO3/1:1 MeOH/THF. Reaction 4: TsOH/NaNO2/NaN3/THF/0 °C.
