Fig 2.
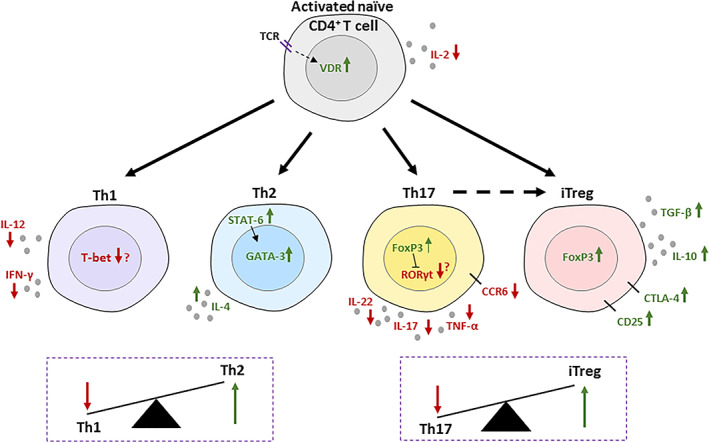
Immunomodulatory effects of 1,25D on CD4+ T cells. T‐cell‐receptor signaling induces an upregulation in the vitamin D receptor (VDR), which is stabilized from degradation by 1,25D binding. IL‐2 production is suppressed by 1,25D signaling after T‐cell activation. Th1‐ and Th17‐cell differentiation is suppressed by vitamin D3 signaling along with characteristic, inflammatory cytokine production. CCR6 on Th17 cells is also suppressed, preventing homing to tissues. Downregulation of transcription factors T‐bet and RORγt is less consistently reported. Th2‐cell differentiation is widely thought to be increased by 1,25D, although this is dependent on conditions. 1,25D causes upregulation of IL‐4 and STAT‐6, which increases GATA‐3. Overall, the Th1/Th2 balance is in the favor of Th2 cells. Similarly, the Th17/iTreg balance favors Tregs with the addition that Th17 cells, additionally suppressed by FoxP3 upregulation, can convert to a Treg phenotype. 1,25D upregulates IL‐10, TGF‐β, and inhibitory markers CTLA‐4 and CD25 to promote an anti‐inflammatory phenotype.
