Abstract
The safety of immunosuppressive treatment in patients with Immune‐Mediated Inflammatory Diseases (IMIDs) during the Coronavirus pandemic is questioned and it is utmost important for public health. We searched studies trough MEDLINE/EMBASE database, including patient with IMID, undergoing immunosuppressive treatment with a positive diagnosis for SARS‐CoV 2. We included 11 studies for the descriptive analysis and 10 studies for the pooled analysis, with a total population of 57 and 53 IMID‐affected SARS‐CoV‐positive patients respectively. Overall no death was reported; 16 patients were hospitalized (30.2%) and only two cases were admitted to Intensive Care Unit (ICU) (3.8%). We found a significant association between the risk of hospitalization and older age (P .03), obesity (P .02), and presence of multi‐comorbidity (P .03). No significant association was found between the risk of hospitalization and the use of biological or conventional DMARDs (respectively P .32 and .26), neither when they are used combined (P .85). We found consistent results in the sub‐analysis of Psoriasis: 10 patients were hospitalized (31.3%) and only one case was admitted to Intensive Care Unit (ICU) (3.1%) Particular attention should be placed for patients with older age, obesity and multi‐comorbidity that are at higher risk of hospitalization.
Keywords: biologic, coronavirus, immune‐mediate inflammatory disease, immunosuppressive drugs, psoriasis
1. INTRODUCTION
Whether patients under biological treatment for dermatologic, rheumatologic, or gastroenterological immune mediated diseases (IMIDs) should stop or continue therapy during Coronavirus pandemic has been a matter of debate. 1 It has been advised that since the rate of respiratory infection during clinical trials with biologics for psoriasis was comparable with placebo and that these drugs should not be withhold. 1 In IBD patients, although, figures suggest that infection rate is low, 2 fear of a worst outcome in immunosuppressed patients has been put forward. 3 Remarkably, in several IMID not undergoing immunosuppressive treatment was detected latent airway inflammation with the technique of fraction exhaled nitric oxide (FeNO) that may contribute to increase COVID‐19 vulnerability synergically with the dysimmunity typical of IMID. 4 , 5 , 6 , 7 The use of some biological drug has been even suggested to ease the severity of lung disease following the infection. 8 , 9 Pooled data on the clinical fate of SARS‐CoV2 infected patients under biologicals were not available in literature to have any clue on this new pandemic. Data on the outcome of the same type of patients from first coronavirus pandemic with SARSCoV1 may help to understand how to manage these cases in the current pandemic. For this reason we performed a systematic review on patients with IMIDs treated with biologics or conventional DMARDs that become infected with SARS‐CoV2 or SARS‐CoV1 and analyzed their clinical outcome.
2. METHODS/LITERATURE SEARCH
We searched studies from the database PubMed MEDLINE and OVID Embase, using the strategy shown in Table 1.
TABLE 1.
MEDLINE and EMBASE search strategy
| # | Searches |
|---|---|
| 1 | Middle east respiratory syndrome coronavirus [mesh] |
| 2 | Severe acute respiratory syndrome [mesh] |
| 3 | SARS virus [mesh] |
| 4 | Betacoronavirus [mesh] |
| 5 | COVID‐19 [word text] |
| 6 | or/1–5 |
| 7 | Immunosuppressive agents [pharmacological action] |
| 8 | Immunosuppression [mesh] |
| 9 | Psoriasis [word text] |
| 10 | Psoriatic arthritis [word text] |
| 11 | Transplants/therapy [mesh] |
| 12 | Transplants/transplantation [mesh] |
| 13 | biologic [word text] |
| 14 | Infliximab/therapeutic use [mesh] |
| 15 | Adalimumab/therapeutic use [mesh] |
| 16 | Steroids/drug therapy [mesh] OR |
| 17 | Steroids/pharmacology [mesh] |
| 18 | Steroids/therapeutic use [mesh] |
| 19 | Ustekinumab [mesh] |
| 20 | guselkumab [supplementary concept] |
| 21 | risankizumab [supplementary concept] |
| 22 | anti‐IL 23 [word text] |
| 23 | Ixekizumab [supplementary concept] |
| 24 | Secukinumab [supplementary concept] |
| 25 | Brodalumab [supplementary concept] |
| 26 | Bimekizumab [supplementary concept] |
| 27 | or/7‐26 |
| 28 | animals/not humans/ |
| 29 | 27 not 28 |
| 30 | and/6,29 |
| 31 | limit 30 to (clinical conference or editorial) |
| 32 | 30 not 31 |
| 33 | remove duplicates from 32 |
Note: Database(s): NCBI PubMed Central (PMC) MEDLINE(R) U.S. National Institutes of Health's National Library Medicine; Embase Classic+Embase 1947 to May 14, 2020 Search Strategy run on May 14, 2020.
Two physicians independently (Federica Giuliani and Giulio Gualdi) proceeded to abstracts screening, according to inclusion and exclusion criteria, examined full‐text articles to determine eligibility and discussed disagreements to reach consensus. We listed excluded studies and primary reasons for exclusion (Figure 1), then we extracted data from reports. We also check the references of the included studies.
FIGURE 1.
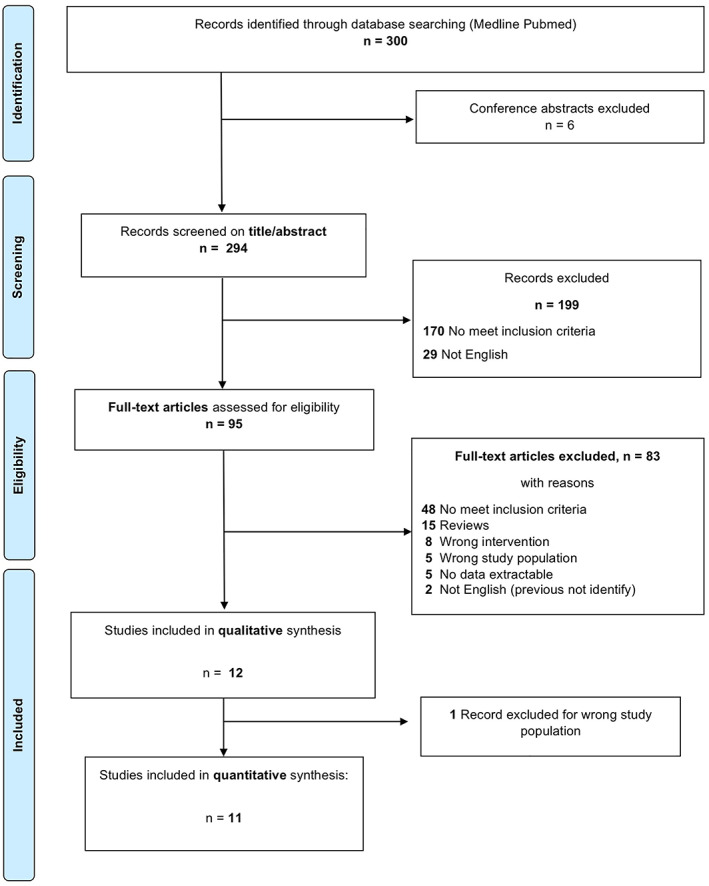
Flow‐diagram chart of study selection and inclusion
We included case report, case series, letter, review, register, prospective, and retrospective studies, in English language and Human. We excluded conference abstract.
We considered the outcomes “hospitalization,” “admission in Intensive Care Unit” and “death.”
We included adult and pediatric patients with a confirm diagnosis of Coronavirus infection made by naso/oro‐pharyngeal swab for RT‐PCR, affected by dermatologic, rheumatologic, and gastroenterological IMID, undergoing immunosuppressive therapy, including biologic and conventional DMARDs (as listed in Table 1). We excluded type of Psoriasis other than plaque psoriasis and psoriatic arthritis.
2.1. Statistical analysis
Individual patient data were pooled from 10 reports. 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 Categorical variables are described as absolute frequencies and percentages and continuous variables are presented as mean values ± SD (SD). Fisher and t‐test were used to compare variables between subjects with or without hospitalization. A two‐sided P value <.05 was considered statistically significant. Statistical analyses were performed by SPSS version 20.0 (IBM Inc., Illinois).
2.2. Missing data
We excluded the unique study 20 that contributed with one patient affected by SARS‐CoV 1 infection, to avoid selection bias.
We excluded four patients from three reports 14 , 15 , 17 for the comorbidity descriptive and pooled analysis because of unclear/missing data.
The study by Monti et al 21 included only mean values of the four patients included, without individual data, and was thus accordingly excluded in the pooled analysis.
3. RESULTS
3.1. Search results
We identified through database searching 300 records: 6 have been excluded because duplicates or conference abstract; 294 records have been screened for titles and abstracts; then we excluded 199 records and we assessed 95 full text articles for eligibility. Of these, 83 full text articles were excluded and 12 records were included for qualitative synthesis. We extract data for a quantitative analysis of 11 complete records. For further descriptions see the study flow‐diagram (Figure 1).
3.2. Population characteristics of included studies
We included 11 reports 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 21 for quantitative descriptive analysis, with a total population of 57 SARS‐CoV 2 confirmed‐infected patients, with an average age of 49.2 years (SD 17.6), and an equally gender distribution (29M, 50.8%; Table 2). Among IMIDs population, 32 had a Psoriasis diagnosis and 4 had also psoriatic arthritis; 20 patients suffered from Inflammatory Bowel Disease (IBD), with 12 Crohn's Diseases (CD), and 8 Ulcerative Colitis (UC). One patient has together Psoriasis and CD. Three patients had Rheumatoid Arthritis, one Ankylosing Spondylitis and one Sjögren Syndrome (SS).
TABLE 2.
Reports selected and included for data analysis and demographic characteristic of study population
| Reports | Evidence levels | IMID | Duration of disease (years) | Patients | Age (mean/SD) | Sex | Comorbidity | Previous immunosoppressor | Immunosuppressive therapy before/during infection | Coronavirus infection | Symptoms/diagnosis | Continued/stopped immunosuppressor | Hospitalization | ICU | Death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Balestri, R (2020) Dermatol Therapy | 5 | Psoriasis | 4 | 1 | 55 | M | No | cDMARDs, Adalimumab | Ixekizumab | COVID‐19 | Asymptomatic | Continued | None | None | None |
| Benhadou, F (2020) JEADV | 5 | Psoriasis | 20 | 1 | 40 | F | Ehlers‐Danlos Syndrome | CsA, MTX | Guselkumab | COVID‐19 | Fever (39.4°C), cough, myalgia, fatigue, shortness of breath | Continued | None | None | None |
| Conti, A (2020) JEADV | 4 | Psoriasis | — | 2 | 64 (2.8) | M | hypertension, dyslipidemia, AMI, diabetes, IRC | — | Guselkumab Ustekinumab | COVID‐19 | ARDS, asthenia, anosmia ageusia | 50% stopped | 50% | 50% | None |
| Damiani, G (2020) JEADV | 4 | PSO, PsA | >1 | 22 | 57.7 (12.5) | 72.7% M | Hypertension, diabetes, obesity, CCD | — |
Anti‐IL12/23 31.8% Anti‐IL17 31.8% Anti‐TNFα (biosimilar) 22.7% Anti‐TNFα 9% |
COVID‐19 | Fever, anosmia, ageusia, astenia, cough | 4.5% stopped | 22.7% | None | None |
| Gisondi, P (2020) BJD | 4 | Psoriasis | — | 4 | 61 (9.8) | 50% M | Hypertension, diabetes, obesity | — | Ustekinumab 25% Adalimumab 25% Etanercept 25% Secukinumab 25% | COVID‐19 | 75% interstitial pneumonia | — | 75% | None | None |
| Messina, F (2020) JEADV | 5 | PSO, PsA | 14 | 1 | 32 | F | CD | cDMARDs, anti‐TNFα, anti‐IL17, anti‐IL12/23 | Guselkumab MTX | COVID‐19 | Fever (37.4°C), mild rhinorrhea | MTX stopped; Guselkumab postponed | None | None | None |
| Nasiri, S (2020) J Dermatolog Treat | 5 | Psoriasis | 1 | 73 | M | — | — | CsA MTX | COVID‐19 | Fever, malaise, and dry cough | MTX stopped stopped & restarted CsA | Yes | None | None | |
| Emmi, G (2020) Autoimmun Rev | 5 | Sjögren Syndrome | — | 1 | 68 | F | — | — | Prednisone + HCQ Tocilizumab (ICU) | COVID‐19 | Fever, dry cough, fatigue, dyspnea, interstitial pneumonia | cDMARDs increased doses Tocilizumab started in ICU | Yes | Yes | None |
| Monti, S (2020) Ann Rheum Disease | 4 | AR, SpA/PsA | — | 4 | 58 | 4 F | Hypertension | — | Etanercept 50% Abatacept 25% Tofacitinib 25% + cDMARDs | COVID‐19 | Fever, fatigue, anosmia/dysgeusia, cough, rhinorrhea, myalgia, dyspnea | Stopped | 25% | None | None |
| Allocca, M Clin Gastroent Hepatol | 4 | IBD | — | 14 | 39.9 (10.4) | 28.6% M | Renal transplantation, hypertension, obesity, arthritis, other | — | Infliximab 42.9% Ustekinumab 14.3% Adalimumab 14.3% cDMARDs 35.7% Vedolizumab 7.1% | COVID‐19 | — | — | 35.7% | None | None |
| Turner, D (2020) JPGN | 4 | IBD | 3.2 | 6 | 16.5 (2) | 50% M | cardiovascular disease | cDMARDs infliximab | Infliximab 33.3% Vedolizumab 16.6% cDMARDs 66.6% | COVID‐19 | Fever, cough, fatigue, anosmia, ageusia, rhinitis, mild chest pain | Continued | None | None | None |
Note: For evidence levels we used quality rating scheme for studies and other evidence modified from the Oxford Centre for evidence‐based medicine; 4: case series with or without intervention; cross‐sectional study, 5: opinion of respected authorities; case reports.
Abbreviations: bDMARDs, biologic disease‐modifying anti‐rheumatic drugs; CCD, cardio‐cerebrovascular disease; CD, Crohn disease; cDMARDs, conventional disease‐modifying anti‐rheumatic drugs; CPP, chronic paranoid psychosis; HCQ, hydrossichloroquine; ICU, intensive care unite; IMID, immune‐mediated inflammatory disease; PE, pulmonary embolism; PsA, psoriatic arthritis; PSO, psoriasis; PSC, primary sclerosing cholangitis; UC, ulcerative colitis.
From a total population of 53 patients (see Section 2.2), 13 had more than one comorbidity (24.5%). Comorbidities were: hypertension (26.4%), diabetes (9.4%), cardio‐cerebrovascular disease (9.4%), obesity (11.3%), and other (26.4%).
3.3. Intervention characteristics of included studies
On a total population of 57 patients, 50 patients were treated with a biologic therapy (87.7%) and 1 patient with a JAK inhibitor (Tofacicitinib, 1.7%). Six patients used conventional DMARDs alone (10.5%) and 10 patients were treated with combined biologic and conventional DMARDs (17.5%; see Table 2).
3.4. Pooled analysis
We conducted a pooled analysis on 10 reports 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 regarding 53 patients (see Section 2.2 for population details) affected by SARS‐CoV 2, with a previous diagnosis of IMIDs, treated with a biologic and/or conventional DMARDs, with the objective to assess how the drug‐induced immunosuppressive status could impact the severity of the SARS‐CoV infection clinical course. We also analyze the association between demographic patients characteristic and the outcomes “hospitalization” (Table 3).
TABLE 3.
Clinical and therapeutic characteristics of hospitalized and not‐hospitalized patients with IMID and SARS‐CoV 2 infection
| Overall n = 53 | Hospitalized n = 16 (30.2%) | Not‐hospitalized n = 37 (69.8%) | P‐value | |
|---|---|---|---|---|
| Age (years), mean ± SD | 49.2 ± 17.6 | 56.6 ± 7.9 | 44.9 ± 6.0 | .03 |
| Gender (M), n(%) | 29 (50.9) | 8 (50.0) | 21 (56.8) | .65 |
| Diabetes, n(%) | 5 (10.2) a | 3 (23.1) b | 2 (5.6) c | .07 |
| Hypertension, n(%) | 13 (26.5) a | 6 (46.2) b | 7 (19.4) c | .06 |
| Obesity, n(%) | 6 (12.2) a | 4 (30.8) b | 2 (5.5) c | .02 |
| Cardiocerebrovascular diseases, n(%) | 6 (12.2) a | 1 (7.7) b | 5 (13.9) c | .55 |
| Other, n(%) | 10 (20.4) a | 3 (23.1) b | 7 (19.4) c | .78 |
| >1 comorbidity, n(%) | 12 (24.5) a | 6 (46.2) b | 6 (16.7) c | .03 |
| bDMARDs d , n(%) | 41 (77.4) | 11 (68.8) | 30 (81.1) | .32 |
| Anti‐IL23, n(%) | 3 (5.7) | 1 (6.3) | 2 (5.4) | .90 |
| Anti‐IL12/23, n(%) | 11 (20.8) | 3 (18.8) | 8 (21.6) | .81 |
| Anti‐TNFα, n(%) | 18 (34.0) | 4 (25.0) | 14 (37.8) | .36 |
| Anti‐IL17, n(%) | 9 (17.0) | 3 (18.8) | 6 (16.2) | .82 |
| cDMARDs d , n(%) | 6 (11.3) | 3 (18.8) | 3 (8.1) | .26 |
| bDMARDs + cDMARDs, n(%) | 6 (11.3) | 2 (12.5) | 4 (10.8) | .85 |
Note: Values are expressed as mean ± SD or n (%).
Abbreviations: bDMARDs, biologic disease‐modifying antirheumatic drugs; cDMARDs, conventional disease‐modifying antirheumatic drugs; M, male.
Missing data for comorbidity modify the total population analyzable on a total population of 49 patients.
Missing data for comorbidity modify the total population analyzable on a total population of 13 patients.
Missing data for comorbidity modify the total population analyzable on a total population of 36 patients.
Numbers indicates patients on bDMARDs or cDMARDs monotherapy.
No death was reported; 16 patients were hospitalized (30.2%, 95% CI 17.8‐42.6%) and only 2 cases were admitted to Intensive Care Unit (ICU) (3.8%, 95% CI −1.3 to 8.9; see Table 2).
We found a significant association between the risk of hospitalization and older age (P .03), obesity (P .02), and the presence of multiple comorbidity (P .03). A positive trend for the outcome “hospitalization” was observed in diabetic (P .07), and hypertensive (P .06) patients. No significant association was found between the risk of hospitalization and the use of biological or conventional DMARDs (respectively, P .32 and P .26), neither when they are used combined (P .85; see Table 3).
We also conducted a pooled analysis on seven reports regarding 32 patients affected by COVID‐19, with a previous diagnosis of Psoriasis, treated with a biologic and/or conventional DMARDs, with the objective to assess how the drug‐induced immunosuppressive status could impact the severity of the SARS‐CoV infection clinical course. We also analyze the association between demographic patients characteristic and the outcomes “hospitalization” (Table 4).
TABLE 4.
Clinical and therapeutic characteristics of hospitalized and not‐hospitalized psoriatic patients with SARS‐Cov2 infection
| Overall n = 32 | Hospitalized n = 10 (31.3%) | Not‐hospitalized n = 22 (68.7%) | P‐value | |
|---|---|---|---|---|
| Age (years), mean ± SD | 57.6 ± 4.5 | 64.1 ± 6.5 | 54.6 ± 5.8 | .04 |
| Gender (M), n(%) | 22 (68.8) | 7 (70.0) | 15 (68.2) | .91 |
| Diabetes, n(%) | 4 (13.7) a | 3 (37.5) b | 1 (4.8) c | .07 |
| Hypertension, n(%) | 12 (41.4) a | 5 (62.5) b | 7 (33.3) c | .15 |
| Obesity, n(%) | 3 (10.3) a | 1 (12.5) b | 2 (9.5) c | .28 |
| Cardiocerebrovascular diseases, n(%) | 4 (13.7) a | 1 (12.5) b | 3 (14.3) c | .90 |
| Other, n(%) | 4 (13.7) a | 1 (12.5) b | 3 (14.3) c | .90 |
| >1 comorbidity, n(%) | 11 (37.9) a | 5 (62.5) b | 6 (28.6) c | .09 |
| bDMARDs d , n(%) | 30 (93.8) | 9 (90.0) | 21 (95.4) | .55 |
| Anti‐IL23, n(%) | 4 (12.5) | 1 (10.0) | 3 (13.6) | .77 |
| Anti‐IL12/23, n(%) | 8 (25.0) | 2 (20.0) | 6 (27.3) | .66 |
| Anti‐TNFα, n(%) | 9 (28.1) | 2 (20.0) | 7 (31.8) | .49 |
| Anti‐IL17, n(%) | 9 (28.1) | 3 (30.0) | 6 (27.3) | .87 |
| cDMARDs d , n(%) | 1 (3.0) | 1 (10.0) | 0 (0) | .13 |
| bDMARDs + cDMARDs, n(%) | 1 (3.0) | 0 (0) | 1 (4.5) | .49 |
Note: Values are expressed as mean ± SD or n (%).
Abbreviations: bDMARDs, biologic disease‐modifying antirheumatic drugs; cDMARDs, conventional disease‐modifying antirheumatic drugs; M, male.
Missing data for comorbidity modify the total population analyzable on a total population of 29 patients.
Missing data for comorbidity modify the total population analyzable on a total population of 8 patients.
Missing data for comorbidity modify the total population analyzable on a total population of 21 patients.
Numbers indicates patients on bDMARDs or cDMARDs monotherapy.
Also in this group no death was reported; 10 patients were hospitalized (31.3%, 95% CI 15.2‐47.3%) and only one case was admitted to Intensive Care Unit (ICU) (3.1%, 95% CI −2.9 to 9.2%; see Table 1). We observed a significant association between the risk of hospitalization and older age (P .04). A positive trend for the outcome “hospitalization” was found for the variable diabetic (P .07). Even in the psoriatic population, no significant association was found between the risk of hospitalization and the use of biological or conventional DMARDs (respectively P .55 and P .13), neither when they are used combined (P .49; see Table 4).
4. DISCUSSION
SARS Coronavirus (SARS‐CoV1) caused an outbreak of severe acute respiratory syndrome in 2002. This SARS was characterized by an atypical acute, community‐acquired pneumonia. The epidemic ended in July 2003, leaving a total of 8096 infected patients and 774 deaths (9.5%) in over 30 countries. 22 , 23 , 24
In December 2019 a new coronavirus infection called SARS‐CoV 2, causing a new diseases. Named COVID‐19, was recognized in China and quickly spread to countries in and outside Asia becoming pandemic.
While in some individuals, the COVID‐19 disease remains asymptomatic, albeit infective, other individuals present severe complications. 25 Around 15% of patients develop severe pneumonia and 5% progress to an acute respiratory distress syndrome, septic shock and/or a multiple organ failure, associated with high mortality. 26 Both innate and adaptive immune response were implicated in the severity differential of this disease. 27
A “cytokine storm” following hyper‐activation of the immune system seems to be responsible for this progression. 28 Several cytokines such as IP‐10, MCP‐1 IL‐2, IL‐6, IL7, GM‐CSF, and TNF alpha have been related not only to the severity of the disease but also with the probability of being admitted to the ICU. 29 , 30
In particular high TNF alpha and IL‐6 levels have been described as biomarkers of worse outcome in particularly fragile cancer patients infected with SARSCoV2. 31
Since immunosuppression across multiple cytokine axes has the potential to increase susceptibility, persistence, and reactivation of viral infections, the question for dermatology, rheumatology and gastroenterology was whether to halt therapies for IMIDs patients during this pandemic. The discontinuation of immunosuppressants in IMIDs, however, may lead to disease flares, and severe psychological distress, that sometimes could be more harmful than stopping the therapy for the fear of getting the infection. 32
Moreover disease flare implies systemic inflammation and immunological disruption, two recognized factors responsible for increasing susceptibility to infection and severity of disease. 33
The choice of action is crucial since it can impact on the efficacy, safety of treatment, and quality of patient's life. The approach in the management of anti‐interleukins anti‐cytokines treated patients has been questioned among health care providers dealing with IMIDs in various areas of medicine. 1 , 3 , 34 , 35
We focused our review on the evaluation of immunosuppressants impact during COVID‐19 outbreak in the understudied IMID population.
In our pooled analysis we found that 30.2% of SARS‐CoV positive patients undergoing immunosuppressive or immunomodulatory therapy was hospitalized. When we analyzed the subgroup of psoriatic patients under therapy this figure remained stable at 31.3%. In Italy the hospitalization rate in the same period taken in consideration in our study was 16.9%, 36 but a direct comparison is not methodologically correct since the cases collected in our study come from different countries and they represent a selected population, not the totality of IMIDs patients undergoing immunosuppressive therapy. Instead as for the severity of the disease we found that 3.5% of the patients collected in our pooled analysis was admitted to ICU and this is very similar to the rate of ICU admission recorded in the Italian COVID‐19 population. 36
We found that the features that may predict a worse prognosis for IMID treated patients were age, obesity and multi‐comorbidity. Among the comorbidities usually described as being associated with a higher risk of infection and disease severity only diabetes and hypertension showed a tendency towards statistical significance in the patients collected.
This is in line with the literature that demonstrates that, among several clinical features and comorbidities that may predict the severity of the disease and thus the admission to ICU, age was the strongest of all. 37
As for the role of therapy, although the numbers are very small, our pooled analysis showed that nor classical DMARDs neither biological previous treatment, generates a higher risk of hospitalization in IMID patients and more specifically to psoriatic patients treated with these drugs. We think that this evidence may be helpful in guiding the management of these patients. Moreover this may be in line with the hypothesis that some level of immune modulation may be worthwhile to control the complication of COVID‐19 infection. 38
Although there have been no cases of patients treated with Apremilast included in our review, the evidence is in support of a good safety profile with this drug 39 and probably this is the main reason for their absence. Indeed many authors reported that none of the patients treated with Apremilast developed COVID‐19 related symptoms. 40
We combined the present evidences from this paper with the management suggestion already in place, 41 , 42 and developed an algorithm for the management of patients treated with DMARDs during this pandemic (Figure 2). The main limitation of our pooled analysis is the small sample size, possibly not allowing adequate statistical power, even if representing an insightful description of the now available best knowledge about the topic.
FIGURE 2.
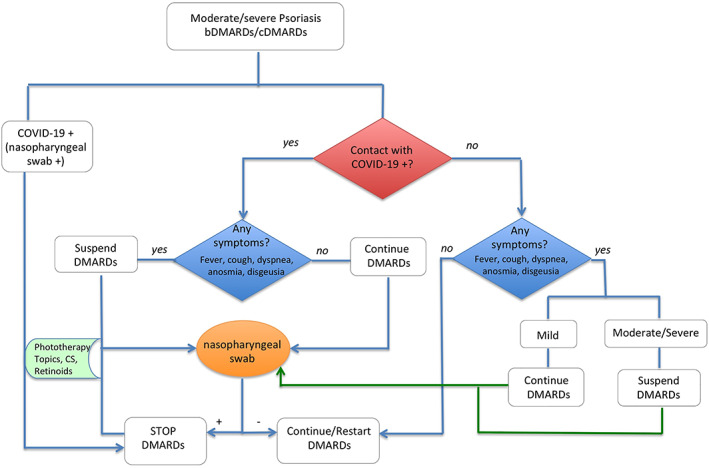
Algorithm proposal for psoriasis therapy management during COVID‐19 era
5. CONCLUSION
Our systematic review and pooled analysis on COVID‐19 infected IMID patients treated with immunosuppressive and immunomodulatory drugs showed that there is no difference in the hospitalization rate for DMARDs users. Particular attention should be placed for patients with older age, obesity and multi‐comorbidity in treatment with these drugs.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Giuliani F, Gualdi G, Amerio P. Effect of immunosuppressive drugs in immune‐mediated inflammatory disease during the coronavirus pandemic. Dermatologic Therapy. 2020;33:e14204. 10.1111/dth.14204
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Lebwohl M, Rivera‐Oyola R, Murrell DF. Should biologics for psoriasis be interrupted in the era of COVID‐19? Asian Pac J Cancer Prev. 2020;82(5):1217‐1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brenner EJ UR, Colombel JF, Kappelman MD. SECURE‐IBD database public data update. covidibd.org. 2020.
- 3. Monteleone G, Ardizzone S. Are patients with inflammatory bowel disease at increased risk for Covid‐19 infection? J Crohns Colitis. 2020;jjaa061. 10.1093/ecco-jcc/jjaa061. [online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Damiani G, Pacifico A, Rizzi M, et al. Patients with psoriatic arthritis have higher levels of FeNO than those with only psoriasis, which may reflect a higher prevalence of a subclinical respiratory involvement. Clin Rheumatol. 2020;10. 10.1007/s10067-020-05050-2 [DOI] [PubMed] [Google Scholar]
- 5. Damiani G, Pigatto PDM, Marzano AV, et al. Malar rash is a predictor of subclinical airway inflammation in patients with systemic lupus erythematosus: a pilot study. Clin Rheumatol. 2019;38(9):2541‐2546. [DOI] [PubMed] [Google Scholar]
- 6. Damiani G, Radaeli A, Olivini A, Calvara‐Pinton P, Malerba M. Increased airway inflammation in patients with psoriasis. Br J Dermatol. 2016;175(4):797‐799. [DOI] [PubMed] [Google Scholar]
- 7. Malerba M, Ragnoli B, Buffoli L, et al. Exhaled nitric oxide as a marker of lung involvement in Crohn's disease. Int J Immunopathol Pharmacol. 2011;24(4):1119‐1124. [DOI] [PubMed] [Google Scholar]
- 8. Jamilloux Y, Henry T, Belot A, et al. Should we stimulate or suppress immune responses in COVID‐19? Cytokine and anti‐cytokine interventions. Autoimmun Rev. 2020;19(7):102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pacha O, Sallman MA, Evans SE. COVID‐19: a case for inhibiting IL‐17? Nat Rev Immunol. 2020;20(6):345‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Balestri R, Rech G, Girardelli CR. SARS‐CoV‐2 infection in a psoriatic patient treated with IL‐17 inhibitor. J Eur Acad Dermatol Venereol. 2020;34(8):e357‐e358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Benhadou F, Del Marmol V. Improvement of SARS‐CoV2 symptoms following Guselkumab injection in a psoriatic patient. J Eur Acad Dermatol Venereol: JEADV. 2020;34:e363‐e364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Conti A, Lasagni C, Bigi L, Pellacani G. Evolution of COVID‐19 infection in four psoriatic patients treated with biological drugs. J Eur Acad Dermatol Venereol. 2020;34(8):e360–e361. 10.1111/jdv.16587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Damiani G, Pacifico A. Biologics increase the risk of SARS‐ CoV‐2 infection and hospitalization, but not ICU admission and death: real‐life data from a large cohort during RED‐ZONE declaration. 2020:e13475. [DOI] [PMC free article] [PubMed]
- 14. Emmi G, Bettiol A, Mattioli I, et al. SARS‐CoV‐2 infection among patients with systemic autoimmune diseases. Autoimmun Rev. 2020;19:102575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gisondi P, Facheris P, Dapavo P, et al. The impact of COVID‐19 pandemic on patients with chronic plaque psoriasis being treated with biologic therapy: the northern Italy experience. Br J Dermatol. 2020;183:373‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Messina F, Piaserico S. SARS‐CoV‐2 infection in a psoriatic patient treated with IL‐23 inhibitor. J Eur Acad Dermatol Venereol: JEADV. 2020;34:e254‐e255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nasiri S, Araghi F, Tabary M, Gheisari M, Mahboubi‐Fooladi Z, Dadkhahfar S. A challenging case of psoriasis flare‐up after COVID‐19 infection. J Dermatolog Treat. 2020;31(5):448‐449. 10.1080/09546634.2020.1764904 [DOI] [PubMed] [Google Scholar]
- 18. Turner D, Huang Y, Martin‐de‐Carpi J, et al. Corona Virus Disease 2019 and Paediatric Inflammatory Bowel Diseases: Global Experience and Provisional Guidance (March 2020) from the Paediatric IBD Porto Group of European Society of Paediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2020;70(6):727‐733. 10.1097/MPG.0000000000002729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Allocca M, Fiorino G, Zallot C, et al. Incidence and patterns of COVID‐19 among inflammatory bowel disease patients from the Nancy and milan cohorts. Clin Gastroenterol Hepatol. 2020;18:2134‐2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mok CC, Ying KY. Lupus pneumonitis or severe acute respiratory syndrome? Lupus. 2004;13(7):549‐553. [DOI] [PubMed] [Google Scholar]
- 21. Monti S, Balduzzi S, Delvino P, Bellis E, Quadrelli VS, Montecucco C. Clinical course of COVID‐19 in a series of patients with chronic arthritis treated with immunosuppressive targeted therapies. Ann Rheum Dis. 2020;79(5):667‐668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. World Health Organization . Summary Table of SARS Cases by Country, November 1, 2002‐August 7, 2003. Geneva, Switzerland: The World health Organization, 2003. [Google Scholar]
- 23. Hui DS, Azhar EI, Kim YJ, Memish ZA, Oh MD, Zumla A. Middle East respiratory syndrome coronavirus: risk factors and determinants of primary, household, and nosocomial transmission. Lancet Infect Dis. 2018;18(8):e217‐e227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leong HN, Chan KP, Oon LL, et al. Clinical and laboratory findings of SARS in Singapore. Annals of the Academy of Medicine, Singapore. 2006;35(5):332‐339. [PubMed] [Google Scholar]
- 25. Rizzo P, Vieceli Dalla Sega F, Fortini F, Marracino L, Rapezzi C, Ferrari R. COVID‐19 in the heart and the lungs: could we "notch" the inflammatory storm? Basic Res Cardiol. 2020;115(3):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cao X. COVID‐19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20(5):269‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Azkur AK, Akdis M, Azkur D, et al. Immune response to SARS‐CoV‐2 and mechanisms of immunopathological changes in COVID‐19. Allergy. 2020;75:1564‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Paces J, Strizova Z, Smrz D, Cerny J. COVID‐19 and the immune system. Physiol Res. 2020;69(3):379‐388. 10.33549/physiolres.934492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tian J, Yuan X, Xiao J, et al. Clinical characteristics and risk factors associated with COVID‐19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21:893‐903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Amerio P, Prignano F, Giuliani F, Gualdi G. COVID‐19 and psoriasis: should we fear for patients treated with biologics? 2020. [DOI] [PMC free article] [PubMed]
- 33. Coletto LA, Favalli EG, Caporali R. Psoriasis and psoriatic arthritis: How to manage immunosuppressants in COVID ‐19 days. Dermatologic Therapy. 2020;33(4):e13415. 10.1111/dth.13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kansal NK. COVID‐19, syphilis, and biologic therapies for psoriasis and psoriatic arthritis: A word of caution. Journal of the American Academy of Dermatology. 2020;82(6):e213. 10.1016/j.jaad.2020.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schulze‐Koops H, Krueger K, Specker C. No advice to discontinue antirheumatic therapy for non‐medical reasons in light of SARS‐CoV‐2. Response to: ‘Treatment adherence of patients with sytemic rheumatic diseases in COVID‐19 pandemic’ by Fragoulis et al. Ann Rheum Dis. 2020;217987. 10.1136/annrheumdis-2020-217987. [DOI] [PubMed] [Google Scholar]
- 36.(ISS) ISdS. Epidemia COVID‐19 Aggiornamento nazionale 7 maggio 2020—ore 16:00. 2020.
- 37. Galloway JB, Norton S, Barker RD, et al. A clinical risk score to identify patients with COVID‐19 at high risk of critical care admission or death: an observational cohort study. J Infect. 2020;81:282‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Md Insiat Islam R. Current drugs with potential for treatment of COVID‐19: a literature review. J Pharm Pharm Sci. 2020;23(1):58‐64. [DOI] [PubMed] [Google Scholar]
- 39. Bridgewood C, Damiani G, Sharif K, et al. Rationale for evaluating PDE4 inhibition for mitigating against severe inflammation in COVID‐19 pneumonia and beyond. Israel Med Ass J: IMAJ. 2020;22(6):335‐339. [PubMed] [Google Scholar]
- 40. Melis D, Mugheddu C, Sanna S, Atzori L, Rongioletti F. Clinical efficacy, speed of improvement and safety of apremilast for the treatment of adult Psoriasis during COVID‐19 pandemic. Dermatologic Therapy. 2020;33(4):e13722. 10.1111/dth.13722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Di Lernia V. Biologics for psoriasis during COVID‐19 outbreak. J Am Acad Dermatol. 2020;82(6):e217‐e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Price KN, Frew JW, Hsiao JL, Shi VY. COVID‐19 and immunomodulator/immunosuppressant use in dermatology. Rheumatol Int. 2020;82(5):e173‐e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


