Abstract
Objective
To describe clinical characteristics of pregnant and postpartum women with severe COVID‐19 in Brazil and to examine risk factors for mortality.
Design
Cross‐sectional study based on secondary surveillance database analysis.
Setting
Nationwide Brazil.
Population or sample
978 Brazilian pregnant and postpartum women notified as COVID‐19 Acute Respiratory Distress Syndrome (ARDS) cases with complete outcome (death or cure) up to 18 June 2020.
Methods
Data was abstracted from the Brazilian ARDS Surveillance System (ARDS‐SS) database. All eligible cases were included. Data on demographics, clinical characteristics, intensive care resources use and outcomes were collected. Risk factors for mortality were examined by multivariate logistic regression.
Main outcome measures
Case fatality rate.
Results
We identified 124 maternal deaths, corresponding to a case fatality rate among COVID‐19 ARDS cases in the obstetric population of 12.7%. At least one comorbidity was present in 48.4% of fatal cases compared with 24.9% in survival cases. Among women who died, 58.9% were admitted to ICU, 53.2% had invasive ventilation and 29.0% had no respiratory support. The multivariate logistic regression showed that the main risk factors for maternal death by COVID‐19 were being postpartum at onset of ARDS, obesity, diabetes and cardiovascular disease, whereas white ethnicity had a protective effect.
Conclusions
Negative outcomes of COVID‐19 in this population are affected by clinical characteristics but social determinants of health also seem to play a role. It is urgent to reinforce containment measures targeting the obstetric population and ensure high quality care throughout pregnancy and the postpartum period.
Tweetable abstract
A total of 124 COVID‐19 maternal deaths were identified in Brazil. Symptoms onset at postpartum and comorbidities are risk factors.
Keywords: COVID‐19, health services accessibility, health status indicators, maternal death
Tweetable abstract
A total of 124 COVID‐19 maternal deaths were identified in Brazil. Symptoms onset at postpartum and comorbidities are risk factors.
Introduction
COVID‐19 is an infection with predominant respiratory features caused by the novel coronavirus SARS‐CoV‐2. The disease rapidly spread worldwide and was declared a global pandemic on 11 March 2020, by the World Health Organization (WHO). By 20 July 2020, COVID‐19 had affected more than 14 million people in 188 territories, with more than 608 000 deaths. 1 Despite the magnitude of incidence and mortality, how the infection impacts pregnancy and whether pregnancy and the postpartum period would lead to more vulnerability remain uncertain. 2 , 3
Initial case series from China did not identify increased risk of adverse outcomes among obstetric patients when compared with the general population, and no maternal deaths were reported. 4 , 5 However, physiological adaptations in normal pregnancies, mainly cardio‐respiratory and immune, are known to increase the susceptibility of pregnant women to several infectious agents, viral pneumonia in particular. 6 Thus, clinicians worldwide remained worried about the impact of COVID‐19 in this population. Subsequent data emerging from Europe and North America also concluded that pregnant women were at no increased risk of severe COVID‐19 or death. 3 , 7 , 8 More recently, further studies reported higher risk of ICU admission and mechanical ventilation during pregnancy, 9 , 10 and the first cases of near miss and maternal deaths have emerged from Iran, US, UK, France, Mexico and Spain. 7 , 8 , 9 , 11 , 12 , 13 , 14 , 15 , 16
In Brazil, approximately 2 months after the first official COVID‐19 case was reported, 1700 deaths among the general population and five maternal deaths had already been documented, raising concerns that perhaps the pandemic in low‐ and middle‐income countries could pose additional risks for pregnant women. 17 It was hypothesised that higher birth rates, worse population health status and poor quality of obstetric care, now competing with constraints resulting from the pandemics management, would contribute to an increase in the absolute number of deaths and also the case fatality rate. 17
The present analysis continues the initial investigations 17 , 18 , 19 of our group using data from the Brazilian Ministry of Health Acute Respiratory Distress Syndrome (ARDS) Surveillance System (ARDS‐SS) to describe clinical characteristic and to examine risk factors for death among COVID‐19 cases during pregnancy and the postpartum period in Brazil.
Materials and methods
This is a secondary database, with cross‐sectional analysis of the ARDS‐SS. Data were extracted from the ARDS‐SS, which comprises mandatory notifications of all ARDS cases in the country from public and private units in all states. ARDS‐SS was established in 2009 as a response to the H1N1 pandemic, and specific fields to track pregnant and postpartum women have been available since then. 20 New specific fields were incorporated to the ARDS Notification Form to gather information on COVID‐19 cases and are included in the database as well. Anonymised data is made publicly available by the Ministry of Health. Data were abstracted in 18 June 2020 using the following inclusion criteria: (i) pregnant or postpartum women; (ii) confirmed (nasopharyngeal RT‐PCR) SARS‐CoV2 infection or confirmed COVID‐19 case based on Brazilian Ministry of Health case definition; (iii) final outcome (death or cure) recorded in the database; (iv) registered from 26 February (date of the first COVID‐19 case in the country) to 18 June 2020. We have been monitoring maternal deaths due to COVID‐19 in Brazil since the beginning of the pandemic. So far we have published preliminary data on subsets of the present sample as they were available in each publication date, 17 , 18 , 19 , 21 the last one including all 124 fatal cases notified up to 18 June 2020, as well as 854 cases who were eventually cured. 18
Besides ARDS‐SS specific fields indicating whether a case is pregnant or in the postpartum period, we also hand‐searched for mentions of pregnancy or postpartum in an open‐ended field related to other comorbidities or risk factors. Information on gestational age or pregnancy outcome is not routinely collected by the ARDS‐SS (only gestational trimester information is available). A COVID‐19 diagnosis was defined as the final classification of each case in the database according to the epidemiological investigation performed by the notifying unit. Figure 1 presents the case selection process, as well as the proportion of cases for whom a SARS‐CoV‐2 RT‐PCR result was available. Among fatal cases, 95.2% had COVID‐19 laboratory confirmation and 80.6% had SARS‐CoV‐2 RT‐PCR results available (serological antibodies and rapid test were recorded for the other cases). These proportions were 97.4 and 78.7%, respectively, among survivors.
Figure 1.
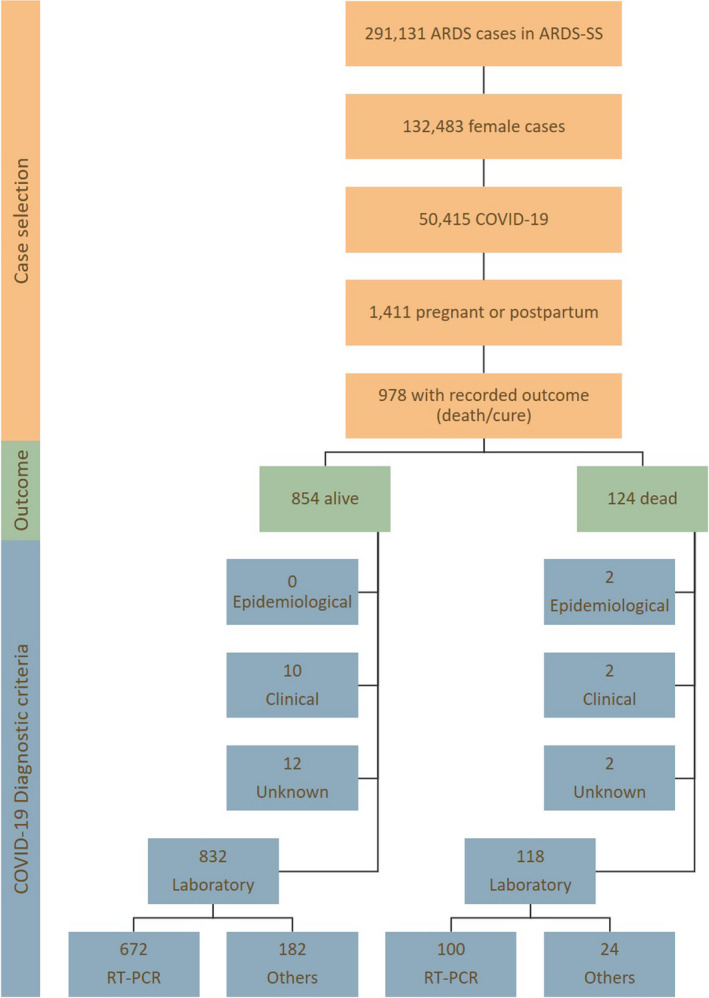
Case selection flowchart.
The main outcome was COVID‐19 case fatality rate among ARDS cases in pregnant and postpartum women and the following variables were analysed for each case: age, ethnicity, pregnancy or postpartum status at notification date, gestational trimester at notification date (for pregnant cases), comorbidities (diabetes, cardiovascular disease, asthma, obesity), ICU admission and respiratory support requirement. Pregnancy or postpartum status and gestational trimester at notification date was assumed to be a proxy for onset timing of symptoms, as the Notification Form collecting this information is usually filled in upon hospital admission due to ARDS. Comorbidity‐related ARDS‐SS fields do not allow identification of gestational or pre‐gestational diabetes and hypertension and did not separate heart diseases from hypertensive disorders; thus, these conditions are grouped under the variables ‘diabetes’ and ‘cardiovascular disease’. For the present analysis, we interpreted missing data as absence of the specific condition or characteristic. This assumption was applicable to comorbidities, ICU admission and respiratory support. Overall missingness for comorbidities on ARDS‐SS has previously described by Baqui et al. 22 for the general population and by Takemoto et al. 18 for the obstetric population.
Core outcome sets and patient involvement requirements are not applicable to this analysis due to its retrospective, secondary database nature. Brazilian ethical regulations do not require Institutional Review Board approval for secondary anonymised data analysis.
Sample size calculation was not performed once we included all eligible cases from the nationwide ARDS‐SS database. STATA 12 was used for statistical analyses. Continuous variables were described using measures of central tendency and dispersion and compared using the Mann–Whitney test. Categorical variables were described using measures of frequency and compared by exact Fisher’s and Chi‐square tests. Multiple logistic regression with a simultaneous entry method was used to explore association of demographic and clinical characteristics with risk of death. Variables with statistically significant differences in hypothesis tests were selected to enter the model (age data was dichotomised as ≤ or >35 years). Statistical significance level was set at 0.05 and all P‐values were two‐tailed.
Results
We identified 978 COVID‐19 cases in pregnant or postpartum women in the ARDS‐SS with recorded outcome. The case fatality rate among women with COVID‐19 ARDS during pregnancy and postpartum was 12.7% (124 deaths). Figure 2 illustrates the distribution of cases within the country, with most cases occurring in São Paulo (SP, n = 359), Rio de Janeiro (RJ, n = 107), Ceará (CE, n = 106), and Amazonas (AM, n = 92). Mortality rate among COVID‐19 maternal ARDS cases in these states was: 14.1, 9.4, 29.0 and 5.8%, respectvely (data not shown).
Figure 2.
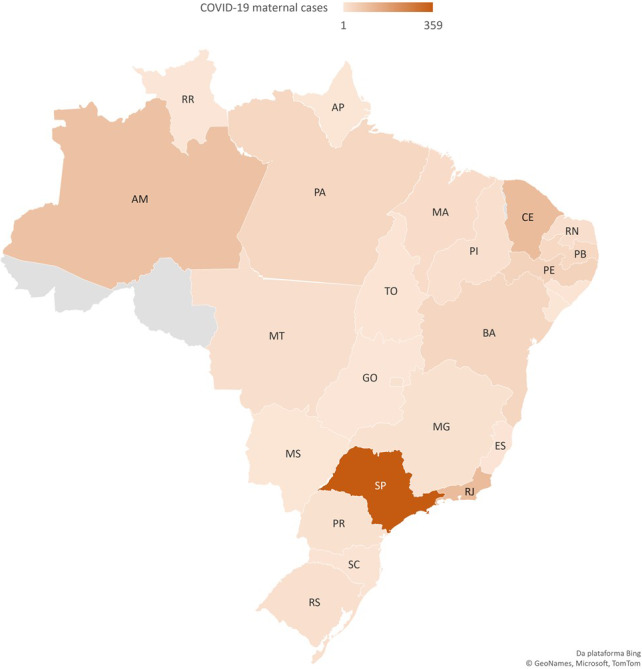
Geographical distribution of pregnant and postpartum cases with COVID‐19 on ARDS‐SS and complete outcome up to 18 June 2020.
Demographic and clinical characteristics of fatal and non‐fatal cases are shown in Table 1. Non‐survivors were older and symptoms onset occurred more frequently postpartum. At least one comorbidity was present in 48.4% of fatal cases compared with 24.9% in survival cases, and the most common was cardiovascular disease, followed by diabetes. Among women who died, 58.9% were admitted to ICU, 53.2% had invasive ventilation and 29.0% had no respiratory support.
Table 1.
Characteristics of COVID‐19 maternal ARDS cases in Brazil (n = 978)
| Death (n = 124) | Cure (n = 854) | P‐value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age, median (IQR) | 32 (25–37) | 30 (24–35) | 0.0039* | ||
| Postpartum | 50 | 40.3 | 174 | 20.4 | <0.0001** |
| Pregnancy | 74 | 59.7 | 680 | 79.6 | |
| 1st trimester | 2 | 2.7 | 55 | 8.1 | 0.1662*** |
| 2nd trimester | 21 | 28.4 | 148 | 21.8 | |
| 3rd trimester | 46 | 62.2 | 449 | 66.0 | |
| Unknown | 5 | 6.7 | 28 | 4.1 | |
| Skin colour/ethnicity | |||||
| White | 23 | 18.5 | 212 | 24.8 | 0.5013*** |
| Black | 5 | 4.0 | 46 | 5.4 | |
| Yellow | 1 | 0.8 | 2 | 0.2 | |
| Brown | 64 | 51.6 | 387 | 45.3 | |
| Indigenous | 1 | 0.8 | 5 | 0.6 | |
| Missing | 30 | 24.2 | 202 | 23.7 | |
| Comorbidities or risk factors | |||||
| Asthma | 5 | 4.0 | 17 | 2.0 | 0.1839** |
| Cardiovascular disease | 26 | 21.0 | 91 | 10.7 | <0.0001** |
| Diabetes | 21 | 16.9 | 65 | 7.6 | <0.0001** |
| Obesity | 12 | 9.7 | 31 | 3.6 | <0.0001** |
| Any comorbidity or risk factor | 60 | 48.4 | 213 | 24.9 | <0.0001** |
| Use of intensive care | |||||
| ICU admission | 73 | 58.9 | 134 | 15.7 | <0.0001** |
| Invasive ventilation | 66 | 53.2 | 32 | 3.7 | <0.0001*** |
| Non‐invasive ventilation | 22 | 17.7 | 197 | 23.1 | |
| No respiratory support | 36 | 29.0 | 625 | 73.2 | |
ICU, intensive care unit; SD, standard deviation.
Mann–Whitney test.
Exact Fisher's test.
Chi‐square test.
The multivariate logistic regression showed that the main risk factors for maternal death by COVID‐19 were being postpartum at onset of ARDS (odds ratio [OR] = 2.48; 95% CI 1.65–3.72), obesity (OR = 2.31; 95% CI 1.10–4.84), diabetes (OR = 1.82; 95% CI 1.01–3.28) and cardiovascular disease (OR = 1.74; 95% CI 1.02–2.94), whereas white ethnicity had a protective effect (OR = 0.58; 95% CI 0.35–0.99) (Table 2).
Table 2.
Multivariate logistic regression analysis of risk factors for maternal death with ARDS due to COVID‐19
| Variables | OR (95% CI) | P‐value |
|---|---|---|
| Postpartum at the time of ARDS notification | 2.481 (1.654–3.720) | <0.0001 |
| Obesity | 2.307 (1.101–4.837) | 0.0268 |
| White ethnicity | 0.585 (0.346–0.991) | 0.0463 |
| Diabetes | 1.817 (1.007–3.278) | 0.0472 |
| Cardiovascular disease | 1.736 (1.024–2.945) | 0.0407 |
ARDS, Acute Respiratory Distress Syndrome; CI, confidence interval; OR, odds ratio.
Classification table 86.9% correctly classified using enter logistic regression method, Constant = −2.316. Area under the receiver operating characteristic (ROC) curve = 0.674, 95% CI 0.643–0.703.
Discussion
Main findings
We identified 978 maternal cases of ARDS and 124 maternal deaths due to COVID‐19 in Brazil. Women in our sample were generally young and the age difference between survivors and non‐survivors was only 2 years, although statistically significant. A significant proportion (51.6%) of women who died from COVID‐19 had no comorbidities or risk factors recorded in the ARDS‐SS database. This seems to indicate that apparently young and healthy women have died due to COVID‐19 complications during pregnancy or just after birth. Among those who had comorbidities, the most common condition was cardiovascular disease followed by diabetes. Similar findings have been documented in a systematic review with non‐pregnant subjects. 23
In our sample, 41.1% of women were not admitted to the ICU and 29.0% did not have records of any type of respiratory support. These findings may indicate that barriers to access intensive care may be playing a role in the overwhelming number of COVID‐19 maternal deaths in Brazil. A US Centers for Disease Control report examining more than 8000 pregnant women with COVID‐19 and 16 cases of maternal deaths identified an increased risk of hospital admission, admission to the ICU and mechanical ventilation in pregnant women, although there was no higher risk of death. 9 Similar findings were also described in Sweden, 10 demonstrating that pregnant women may be more susceptible to COVID‐19 complications, although with adequate and timely intensive care, the survival rate could be similar to non‐pregnant women.
Obstetric cases of ARDS due to COVID‐19 in Brazil are at an increased risk of death if symptoms onset occurs during the postpartum period, they had obesity, diabetes or cardiovascular disease. Similarly, an analysis from Mexico 24 identified that having a comorbidity, particularly diabetes, increases the risk of death among pregnant women with COVID‐19. In our sample, being white was protective against death. Previous findings from UK and US have indicated that pregnant women from ethnic minority groups are at increased risk of adverse outcomes. 8 , 9 Similar findings were expected in Brazil, where racial disparities in the access to healthcare are well documented. 25
Strengths and limitations
Our study is a secondary data analysis of an official nationwide database. In Brazil, ARDS cases due to any cause have been considered mandatory notifications to the Ministry of Health since 2009 and the specific aetiology is recorded, as well as the diagnostic method. Both aspects contribute to the robustness of our analysis in terms of nationwide representativeness and to the higher rate of laboratory‐confirmed SARS‐CoV‐2. Additionally, our findings on risk factors for death are consistent with a previous report from Mexico 24 and also with analysis for the general population. 23 , 26 It is important to highlight that the available data refer to COVID‐19 ARDS cases only and that we do not have information on non‐ARDS COVID‐19 cases in the obstetric population systematically collected in the country. Also, under‐reporting of maternal deaths is a recognised issue in the country. 27
Interpretation
According to our findings, the number of COVID‐19‐related maternal deaths has up to now surpassed the published available combined figures from other countries. 28 To our knowledge, available data on maternal deaths with COVID‐19 worldwide officially consisted of 36 deaths when our data were abstracted on 18 June 2020. It is not possible to rule out globally under‐reported of maternal deaths due to COVID‐19. Although maternal mortality is an important health indicator and is usually strictly monitored worldwide, the data might be incomplete within the pandemic context. Comparatively, during the H1N1 epidemic in 2009, Brazil had 83 maternal deaths due to any influenza pneumonia in a 12‐month period. 29 The number of COVID‐19 maternal deaths in the country in a 3‐month period is 49% higher than the figures for H1N1 for the entire 2009 year.
The COVID‐19 pandemic hit Brazil while the country was still struggling with an unacceptably high maternal death ratio. 29 Despite possible greater susceptibility to severe acute respiratory syndromes in pregnant women in general, it is worth mentioning the significant variation in the number of deaths between different Brazilian States. These findings suggest that negative outcomes in pregnancy during the COVID‐19 pandemic might also be associated with poor quality obstetric care, social risks and barriers to access healthcare, as physiological adaptations and clinical factors are not anticipated to be markedly different across different geographical regions. Notably, Brazil’s federal government actions to contain COVID‐19 pandemic are being recognised as not only ineffective but also endangering. In opposition to universal recommendations, the government has failed to reinforce the need for social isolation or to provide universal screening, as well proper investments in health units and supplies. 30
Currently, the greatest cause of maternal death and near miss in Brazil is hypertension 29 , 31 and inflammatory states have been described as a relevant aetiological hypothesis for both hypertension and pre‐eclampsia. 32 Thus, when hypertension and COVID‐19 simultaneously occur, it is possible to conjecture that inflammatory response may play a role in worsening prognosis, especially during pregnancy. Another possible explanation may be a combination of the country’s high prevalence of overweight and obesity with metabolic syndrome, 33 considering the same inflammatory aspect of immune system response to coronavirus. Obesity was associated with antepartum severe maternal morbidity and may contribute to maternal deaths due its association with pre‐eclampsia. 34 , 35
The prioritisation of COVID‐19 cases throughout the healthcare systems have been described as impacting maternal and neonatal outcomes worldwide. 36 In Brazil, even before the COVID‐19 global pandemic, access to antenatal care faced chronic and complex barriers. 25 , 37 Therefore, barriers to access routine assessment and testing may lead to delays in receiving proper care, potentially contributing to maternal deaths. Additionally, Brazilian caesarean rates are historically high and local data has shown a three times higher risk of maternal death associated with caesarean sections. 38 In the pandemic context, at least one study has already raised awareness of increased risk of adverse and/or severe features of COVID‐19 disease for patients undergoing surgery. 39
The disquieting findings about maternal deaths due to COVID‐19 in Brazil are worrisome, as the country has not been able to control the pandemic and the number of new cases and deaths are still rising. Our data highlight the urgent need for containment measures aimed at the obstetric population, particularly women with high‐risk pregnancies and women postpartum. These measures should include timely and detailed analysis of each COVID‐19‐related maternal death along with COVID‐19‐related maternal near misses (the latter not even auditable through Brazilian health information system). This might allow the production of guidelines and local strategies to improve the patients’ course that are specifically designed for COVID‐19 during pregnancy and postpartum, to enhance maternal and perinatal outcomes.
A call for action in April 2020 already anticipated that women during pregnancy and postpartum might be a vulnerable population for COVID‐19 due not only to biological or clinical factors, but also to social risks. 40 We believe that Brazil is currently facing the tragedy of the aforementioned prediction and that estimating its real dimension can contribute to reversal of the present disaster.
Conclusion
COVID‐19‐related maternal deaths in Brazil have surpassed worldwide combined published figures. Negative outcomes of COVID‐19 in this population are affected by clinical characteristics but social determinants of health and barriers to access proper care seem to play a role. It is urgent to reinforce containment measures targeting the obstetric population and ensure high‐quality care throughout pregnancy and the postpartum period.
Disclosure of interests
None declared. Completed disclosure of interest forms are available to view online as supporting information.
Contribution to authorship
MLST contributed to study conception and study design, conducted literature searches and data extraction, conducted data analysis and interpretation, wrote the first draft of the paper, reviewed and approved the final manuscript. MOM contributed to study conception and study design, conducted literature searches and data extraction, conducted data analysis and interpretation, wrote the first draft of the paper, reviewed and approved the final manuscript. CAB contributed to study conception and study design, conducted data analysis and interpretation, reviewed and provided comments on the first draft, reviewed and approved the final manuscript. RK contributed to study conception and study design, conducted data analysis and interpretation, reviewed and provided comments on the first draft, reviewed and approved the final manuscript. LARS contributed to study conception and study design. LK contributed to study conception and study design, reviewed and approved the final manuscript. EBF contributed to study conception and study design, reviewed and approved the final manuscript. MNP contributed to study conception and study design, conducted data analysis and interpretation, reviewed and provided comments on the first draft, reviewed and approved the final manuscript. CGM contributed to study conception and study design, reviewed and approved the final manuscript. CSGD contributed to study conception and study design, reviewed and approved the final manuscript. ASOM contributed to study conception and study design, conducted literature searches and data collection, conducted data analysis and interpretation, wrote the first draft of the paper, reviewed and approved the final manuscript. MMRA contributed to study conception and study design, wrote the first draft of the paper, reviewed and approved the final manuscript.
Details of ethics approval
According to Brazilian ethics regulatory requirements, secondary analysis of publicly available anonymised data does not require Institutional Review Board ethics approval.
Funding
This research received no specific grant from any funding agency in the public, commercial or not‐for‐profit sectors.
Acknowledgements
The authors would like to thank all members of the Brazilian Group for Studies of COVID‐19 and pregnancy for all efforts in supporting this work.
Supporting information
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Takemoto MLS, Menezes MO, Andreucci CB, Knobel R, Sousa LAR, Katz L, Fonseca EB, Nakamura‐Pereira M, Magalhães CG, Diniz CSG, Melo ASO, Amorim MMR; Brazilian Group for Studies of COVID‐19 and Pregnancy . Clinical characteristics and risk factors for mortality in obstetric patients with severe COVID‐19 in Brazil: a surveillance database analysis. BJOG 2020; 127:1618ߝ1626.
Linked article: This article is commented on by NT Joseph and BJWylie, p. 1627 in this issue. To view this mini commentary visit https://doi.org/10.1111/1471-0528.16521
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. World Health Organization (WHO) . COVID‐19: Situation Report 133. Geneva: WHO; 2020. [Google Scholar]
- 2. Juan J, Gil MM, Rong Z, Zhang Y, Yang H, Poon LCY. Effects of coronavirus disease 2019 (COVID‐19) on maternal, perinatal and neonatal outcomes: a systematic review of 266 pregnancies. Ultrasound Obstet Gynecol. 2020;56:15–‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gajbhiye R, Modi D, Mahale S. Pregnancy outcomes, newborn complications and maternal‐fetal transmission of SARS‐CoV‐2 in women with COVID‐19: a systematic review. medRxiv 2020;2020.04.11.20062356. [Google Scholar]
- 4. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yan J, Guo J, Fan C, Juan J, Yu X, Li J, et al. Coronavirus disease 2019 (COVID‐19) in pregnant women: a report based on 116 cases. Am J Obstet Gynecol 2020;223:111.e1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Somerville LK, Basile K, Dwyer DE, Kok J. The impact of influenza virus infection in pregnancy. Fut Microbiol 2018;13:263–74. [DOI] [PubMed] [Google Scholar]
- 7. Breslin N, Baptiste C, Gyamfi‐Bannerman C, Miller R, Martinez R, Bernstein K, et al. Coronavirus disease 2019 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. American Journal of Obstetrics & Gynecology MFM 2020;2:100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Knight M, Bunch K, Vousden N, Morris E, Simpson N, Gale C, et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS‐CoV‐2 infection in UK: national population based cohort study. Br. J. Med. 2020;369:m2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ellington S, Strid P, Tong VT, Woodworth K, Galang RR, Zambrano LD, et al. Characteristics of women of reproductive age with laboratory‐confirmed SARS‐CoV‐2 infection by pregnancy status – United States, January 22‐June 7, 2020. MMWR Morb Mortal Wkly Rep 2020;69:769–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Collin J, Byström E, Carnahan A, Ahrne M. Pregnant and postpartum women with SARS‐CoV‐2 infection in intensive care in Sweden. Acta Obstet Gynecol Scand 2020;99:819–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blitz MJ, Rochelson B, Minkoff H, Meirowitz N, Prasannan L, London V, et al. Maternal mortality among women with COVID‐19 admitted to the intensive care unit. Am J Obstet Gynecol 2020. https://www.ajog.org/article/S0002‐9378(20)30636‐0/fulltext. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hantoushzadeh S, Shamshirsaz AA, Aleyasin A, Seferovic MD, Aski SK, Arian SE, et al. Maternal death due to COVID‐19 disease. Am J Obstet Gynecol 2020;223:109.e1–109.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lumbreras‐Marquez MI, Campos‐Zamora M, Lizaola‐Diaz de Leon H, Farber MK. Maternal mortality from COVID‐19 in Mexico. Int J Gynecol Obstet 2020;150:266–267. [DOI] [PubMed] [Google Scholar]
- 14. Vallejo V, Ilagan JG. A postpartum death due to coronavirus disease 2019 (COVID‐19) in the United States. Obstet Gynecol 2020;136:52–55. [DOI] [PubMed] [Google Scholar]
- 15. Kayem G, Alessandrini V, Azria E, Blanc J, Bohec C, Bornes M, et al. A snapshot of the Covid‐19 pandemic among pregnant women in France. J Gynecol Obstet Hum Reprod 2020;49:101826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marín Gabriel MA, Cuadrado I, Álvarez Fernández B, González Carrasco E, Alonso Díaz C, Llana Martín I, et al. Multi‐centre Spanish study found no incidences of viral transmission in infants born to mothers with COVID‐19. Acta Paediatr 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ramos Amorim MM, Soligo Takemoto ML, Fonseca EB. Maternal deaths with Covid19: a different outcome from mid to low resource countries? Am J Obstet Gynecol 2020;223:298–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Takemoto MLS, de Menezes MO, Andreucci CB, Nakamura‐Pereira M, Amorim MMR, Katz L, et al. The tragedy of COVID‐19 in Brazil: 124 maternal deaths and counting. Int J Gynecol Obstet 2020;ijgo.13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Takemoto MLS, Menezes MO, Andreucci CB, Knobel R, Sousa LAR, Katz L, et al. Maternal mortality and COVID‐19. J Matern Neonatal Med 2020;16:1–7. [DOI] [PubMed] [Google Scholar]
- 20. Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde . Influenza. In: Health Surveillance Guide. 3rd ed. Brasília‐DF: Ministry of Health; 2019:1–741. [Google Scholar]
- 21. de Santos D, de Menezes MO, Andreucci CB, Nakamura‐Pereira M, Knobel R, Katz L, et al. Disproportionate impact of COVID‐19 among pregnant and postpartum Black Women in Brazil through structural racism lens. Clin Infect Dis 2020. https://academic.oup.com/cid/advance‐article/doi/10.1093/cid/ciaa1066/5877027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baqui P, Bica I, Marra V, Ercole A, van der Schaar M. Ethnic and regional variations in hospital mortality from COVID‐19 in Brazil: a cross‐sectional observational study. Lancet Glob Health 2020;8:E1018–E1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS‐CoV‐2: a systematic review and meta‐analysis. Int J Infect Dis 2020;94:91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martinez‐Portilla RJ, Sotiriadis A, Torres‐Torres J, Christos C, Hawkins‐Villarreal A, Villafan‐Bernal JR, et al. Risk factors for mortality in pregnant women with SARS‐CoV‐2 infection. medRxiv 2020;2020.05.31.20107276. [Google Scholar]
- 25. do Carmo Leal M, da Gama SGN, Pereira APE, Pacheco VE, do Carmo CN, Santos RV. The color of pain: racial iniquities in prenatal care and childbirth in Brazil. Cad Saude Publica 2017;33(Suppl):e00078816. [DOI] [PubMed] [Google Scholar]
- 26. Stefan N, Birkenfeld AL, Schulze MB, Ludwig DS. Obesity and impaired metabolic health in patients with COVID‐19. Nat Rev Endocrinol 2020;16:341–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Szwarcwald CL, Escalante JJC, Rabello Neto DL, Souza PRB Jr, Victora CG. Estimation of maternal mortality rates in Brazil, 2008–2011. Cad Saude Publica 2014;30(Suppl):S1–S12. [DOI] [PubMed] [Google Scholar]
- 28. Nakamura‐Pereira M, Andreucci CB, de Oliveira MM, Knobel R, Takemoto MLS. Worldwide maternal deaths due to COVID‐19: a brief review. Int J Gynecol Obstet 2020;ijgo.13328. https://obgyn.onlinelibrary.wiley.com/doi/full/10.1002/ijgo.13328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brasil. Ministério da Saúde . Departamento de Informática. [Mortality Information System]. SIM‐DATASUS. 2009. [Google Scholar]
- 30. Horton R. Editorial COVID‐19 in Brazil: ‘So what?’. Lancet 2020;6736(20):31095. [Google Scholar]
- 31. Dong L, Tian J, He S, Zhu C, Wang J, Liu C, et al. Possible vertical transmission of SARS‐CoV‐2 from an infected mother to her newborn. JAMA 2020;323:1846–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Harmon AC, Cornelius DC, Amaral LM, Faulkner JL, Cunningham MW Jr, Wallace K, et al. The role of inflammation in the pathology of preeclampsia. Clin Sci 2016;130:409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Horta BL, Barros FC, Lima NP, Assunção MCF, Santos IS, Domingues MR, et al. Maternal anthropometry: trends and inequalities in four population‐based birth cohorts in Pelotas, Brazil, 1982–2015. Int J Epidemiol. 2019;48(Supplement_1):i26–i36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Poorolajal J, Jenabi E. The association between body mass index and preeclampsia: a meta‐analysis. J Mater‐Fetal Neonatal Med 2016;29:3670–6. [DOI] [PubMed] [Google Scholar]
- 35. Siddiqui A, Azria E, Howell EA, Deneux‐Tharaux C, Langer B, Dupont C, et al. Associations between maternal obesity and severe maternal morbidity: findings from the French EPIMOMS population‐based study. Paediatr Perinat Epidemiol 2019;33(1):7–16. [DOI] [PubMed] [Google Scholar]
- 36. Stumpfe FM, Titzmann A, Schneider MO, Stelzl P, Kehl S, Fasching PA, et al. SARS‐CoV‐2 infection in pregnancy ‐ a review of the current literature and possible impact on maternal and neonatal outcome. Geburtshilfe Frauenheilkd 2020;80(4):380–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Domingues RMSM, de Hartz ZMA, Dias ABM, do Carmo Leal M. Avaliação da adequação da assistência pré‐natal na rede SUS do Município do Rio de Janeiro, Brasil. Cad Saude Publica 2012;28(3):425–37. [DOI] [PubMed] [Google Scholar]
- 38. Esteves‐Pereira AP, Deneux‐Tharaux C, Nakamura‐Pereira M, Saucedo M, Bouvier‐Colle MH, Leal DC, et al. Caesarean delivery and postpartum maternal mortality: a population‐based case control study in Brazil. PLoS One 2016;11(4).e0153396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lei C, Huiguo L, Wei L, Jing L, Kui L, Jin S, et al. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia Chen. Chin J Tuberc Respir Dis 2020;43:1–11. [DOI] [PubMed] [Google Scholar]
- 40. Buekens P, Alger J, Bréart G, Cafferata ML, Harville E, Tomasso G. A call for action for COVID‐19 surveillance and research during pregnancy. Lancet Glob Heal 2020;20:2019–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


