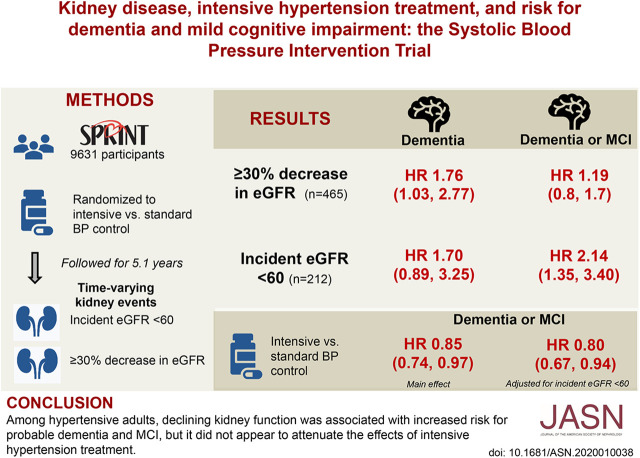
Significance Statement
Intensive treatment of hypertension is beneficial for cardiovascular disease and cognitive function, but at the short-term expense of reduced kidney function. In a randomized trial of intensive versus standard systolic BP lowering, the authors found an association between a large decline in eGFR and increased incidence of probable dementia and mild cognitive impairment, but no link between urinary albumin-to-creatinine ratio and occurrence of dementia or mild cognitive impairment. Decline in eGFR occurred more frequently in the intensive treatment group, but did not modify the beneficial effect of intensive treatment on cognitive function. Among hypertensive adults, declining eGFR may be a marker for those at higher risk for dementia or mild cognitive impairment, independent of the intensity of hypertension treatment.
Keywords: systolic blood pressure, Epidemiology and outcomes, glomerular filtration rate, hypertension
Visual Abstract
Abstract
Background
Intensively treating hypertension may benefit cardiovascular disease and cognitive function, but at the short-term expense of reduced kidney function.
Methods
We investigated markers of kidney function and the effect of intensive hypertension treatment on incidence of dementia and mild cognitive impairment (MCI) in 9361 participants in the randomized Systolic Blood Pressure Intervention Trial, which compared intensive versus standard systolic BP lowering (targeting <120 mm Hg versus <140 mm Hg, respectively). We categorized participants according to baseline and longitudinal changes in eGFR and urinary albumin-to-creatinine ratio. Primary outcomes were occurrence of adjudicated probable dementia and MCI.
Results
Among 8563 participants who completed at least one cognitive assessment during follow-up (median 5.1 years), probable dementia occurred in 325 (3.8%) and MCI in 640 (7.6%) participants. In multivariable adjusted analyses, there was no significant association between baseline eGFR <60 ml/min per 1.73 m2 and risk for dementia or MCI. In time-varying analyses, eGFR decline ≥30% was associated with a higher risk for probable dementia. Incident eGFR <60 ml/min per 1.73 m2 was associated with a higher risk for MCI and a composite of dementia or MCI. Although these kidney events occurred more frequently in the intensive treatment group, there was no evidence that they modified or attenuated the effect of intensive treatment on dementia and MCI incidence. Baseline and incident urinary ACR ≥30 mg/g were not associated with probable dementia or MCI, nor did the urinary ACR modify the effect of intensive treatment on cognitive outcomes.
Conclusions
Among hypertensive adults, declining kidney function measured by eGFR is associated with increased risk for probable dementia and MCI, independent of the intensity of hypertension treatment.
CKD and dementia are common conditions among older adults and frequently co-occur,1,2 suggesting they share a common pathogenesis. Hypertension is a potentially modifiable risk factor for CKD and dementia, affecting more than half of all adults over 60 years of age.3 Clinical guidelines for BP management in adults with CKD focus on the optimal targets for preserving kidney and cardiovascular health.4 Previous randomized trials of intensive versus standard BP targets in patients with CKD did not assess effects on cognitive function.5,6 However, some observational studies in patients with dialysis-dependent CKD have found associations between lower BP with poorer cognition and white matter injury.7,8 Little is known about the relation between BP targets and cognitive function in persons with earlier stages of CKD.
CKD is characterized by the presence of excess albumin in the urine and/or a reduction in eGFR. Although both higher levels of albuminuria, measured by urine albumin-to-creatinine ratio (UACR), and lower levels of eGFR are associated with impaired cognitive function, albuminuria appears to be more consistently associated with cerebral small vessel disease.2,9–11 These observations might suggest that the efficacy or safety of intensive BP targets may differ for adults with CKD characterized by albuminuria versus reduced eGFR alone.
The Systolic Blood Pressure Intervention Trial (SPRINT) was designed to compare the effect of intensive (systolic BP <120 mm Hg) versus standard (systolic BP <140 mm Hg) BP lowering on the incidence of dementia and mild cognitive impairment (MCI).12 Intensive systolic BP lowering was associated with a lower incidence of MCI and the composite outcome of probable dementia and MCI12; however, it was also associated with an increased incidence of eGFR<60 ml/min per 1.73 m2 and AKI.13 In this report, we evaluate the association between baseline and longitudinal changes in eGFR and UACR with incidence of dementia and MCI, and assess whether the effect of intensive versus standard systolic BP lowering on dementia and MCI risk varies according to baseline kidney disease markers.
Methods
Study Participants
The study design and primary outcomes of SPRINT have previously been reported.12,14 In brief, 9361 adult participants with hypertension and increased risk for cardiovascular disease (CVD) were enrolled from 102 clinical sites between November 2010 and March 2013, and randomly assigned to a systolic BP target of <120 mm Hg (intensive treatment) or <140 mm Hg (standard treatment). Individuals were considered to have an increased risk for CVD if they had an eGFR 20–59 ml/min per 1.73 m2, an elevated Framingham risk score, were ≥75 years of age, or had evidence of clinical or subclinical CVD. Major exclusion criteria included diabetes mellitus, proteinuria >1 g/d, polycystic kidney disease, prior stroke, symptomatic heart failure, and left ventricular ejection fraction <35%.
At the baseline visit, participants completed questionnaires ascertaining sociodemographic information, medical and family history, and health behaviors. Trained study personnel recorded height, weight, and BP. Serum creatinine was measured at baseline and at months 1, 3, and every 6 months at the trial’s central laboratory using an enzymatic assay (Roche, Indianapolis, IN). eGFR was calculated using the Modification of Diet in Renal Disease study equation.15 Urine albumin and creatinine were measured from a spot urine sample at baseline, 6 months, and yearly thereafter. Urine albumin was measured using a nephelometric method (Siemens, Tarrytown, NJ). Urine creatinine was measured using the enzymatic assay (Roche).
Measurements
We categorized baseline eGFR as ≥60 ml/min per 1.73 m2 versus <60 ml/min per 1.73 m2, and baseline UACR as <30 mg/g versus ≥30 mg/g. In supplementary analyses, we stratified those with eGFR<60 ml/min per 1.73 m2 into two groups (45–59 ml/min per 1.73 m2 and <45 ml/min per 1.73 m2), and those with UACR≥30 mg/g into two groups (30–299 mg/g and ≥300 mg/g) to assess whether there were graded associations by severity. We categorized longitudinal changes in eGFR and UACR in two ways. Incident eGFR <60 ml/min per 1.73 m2 was a prespecified end point in participants with eGFR≥60 ml/min per 1.73 m2 at baseline, defined as a >30% decrease in eGFR from baseline value, with an end value <60 ml/min per 1.73 m2, confirmed at the next available SPRINT blood draw. Among all SPRINT participants, we also categorized participants according to the presence or absence of ≥30% decrease in eGFR from baseline value. Among participants with UACR<30 mg/g at baseline, incident albuminuria was defined as UACR≥30 mg/g at follow-up.
We categorized smoking status as current, former, or never smokers. We categorized alcohol use as nondrinker (fewer than one drink per month), light drinker (at least one drink per month but fewer than three drinks per week), moderate drinker (at least three drinks per week but fewer than one drink per day), or heavy drinker (one or more drinks per day). We defined CVD as self-report or clinical evidence of coronary artery disease or peripheral arterial disease.14 The number of self-reported physical and mental comorbidities were recorded using the Selim index.16
Outcomes: MCI and Probable Dementia
A detailed description of the cognitive assessment protocol in SPRINT has previously been described.12 Briefly, SPRINT participants received in-person cognitive screening assessments at baseline and follow-up, administered by trained personnel at each site. Participants who could not be assessed in person received a telephone battery.17 When participants scored below prespecified thresholds on the Montreal Cognitive Assessment and the Functional Assessment Questionnaire, an extensive cognitive battery was administered. If a participant died or was unable to communicate, a proxy completed the Dementia Questionnaire. Validated Spanish translations were available when necessary.
An expert panel masked to treatment arm adjudicated cognitive status. Adjudicators reviewed cognitive test scores, proxy functional status reports, standardized measurements of depressive symptoms, self-reported health status, medications, and hospitalizations. Participants were classified into one of four categories: no cognitive impairment, MCI, probable dementia, or cannot classify. Two adjudicators independently reviewed each case using standard diagnostic criteria. The full panel discussed disagreements and made classification decisions by a majority vote. The primary outcome was probable (all-cause) dementia, and secondary outcomes were MCI, defined as two consecutive adjudicated classifications of MCI, and a composite outcome of MCI or probable dementia.
Statistical Analyses
We used Cox proportional hazards regression with the baseline hazard function stratified by clinic site to model the association between baseline eGFR and UACR with probable dementia, MCI, and the composite outcome of probable dementia or MCI. For these analyses, we included treatment group, age, sex, race/ethnicity, education, systolic BP, diastolic BP, use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, smoking status, alcohol use, body mass index, Framingham 10-year cardiovascular risk score, comorbidity (medical and mental summary scores), and Montreal Cognitive Assessment score as covariates. We then examined treatment effect heterogeneity as a function of baseline eGFR and UACR using Cox models including model terms for eGFR (UACR), treatment group, and eGFR (UACR) by treatment group interaction. Subgroup heterogeneity was formally tested using likelihood ratio tests. We modeled the effect of eGFR (<60 versus ≥60 ml/min per 1.73 m2) and UACR (<30 versus ≥30 mg/g) first as binary indicators, and then as continuous variables. For the continuous analyses, we modeled each measure as a linear function with a single break point at 60 ml/min per 1.73 m2 for eGFR and 30 mg/g for UACR.
Next, we conducted analyses to estimate the association between postrandomization changes in eGFR or UACR with the occurrence of cognitive impairment, independent of treatment group. We modeled the association of incident CKD (in participants with eGFR ≥60 ml/min per 1.73 m2 at baseline), incident ≥30% decline in eGFR (all participants), and incident increase in UACR to ≥30 mg/g (participants with UACR<30 mg/g at baseline). For these analyses, we excluded any participants where the development of MCI or dementia preceded changes in kidney function. These analyses included treatment group and the same baseline variables listed above as covariates. Finally, to explore whether change in eGFR attenuates the beneficial effect of intensive treatment on cognitive outcomes, we compared the hazard ratio (HR) for the overall treatment effect to the HR from models that included eGFR decline. All hypothesis tests were two-sided and performed at the 5% level of significance. There were no adjustments for multiple testing.
Results
Participant Characteristics
At baseline, there were 2645 participants (28.2%) with eGFR<60 ml/min per 1.73 m2, and 1730 (18.4%) with UACR≥30 mg/g (Table 1). Compared with participants with eGFR≥60 ml/min per 1.73 m2, participants with eGFR<60 ml/min per 1.73 m2 were older, more likely to be women, white, have lower educational attainment, have CVD, use angiotensin system blockers, and less likely to smoke or use alcohol. They also had more physical comorbidities, fewer mental comorbidities, and lower systolic BP, diastolic BP, body mass index, and lower scores on the Montreal Cognitive Assessment. Compared with participants with UACR<30 mg/g, participants with UACR≥30 mg/g were older, more likely to be black, have lower educational attainment, and have CVD and less likely to smoke or use alcohol. They also had more physical comorbidities, higher systolic BP, and lower scores on the Montreal Cognitive Assessment.
Table 1.
Baseline characteristics of SPRINT participants, stratified by baseline eGFR and UACR
| Variable | eGFR, ml/min per 1.73 m2 | UACR, mg/g | ||||
|---|---|---|---|---|---|---|
| <60 (n=2645) | ≥60 (n=6716) | P Value | ≥30 (n=1730) | <30 (n=7182) | P Value | |
| Age, mean±SD, yr | 71.9±9.3 | 66.3±9.0 | <0.001 | 70.3 (10.1) | 67.4 (9.2) | <0.001 |
| Female sex, n (%) | 1058 (40.0) | 2274 (33.9) | <0.001 | 579 (33.5) | 2568 (35.8) | 0.07 |
| Race/ethnicity, n (%) | <0.001 | 0.04 | ||||
| White | 1777 (67.2) | 3622 (53.9) | 995 (57.5) | 4120 (57.4) | ||
| Black | 637 (24.1) | 2165 (32.2) | 552 (31.9) | 2138 (29.8) | ||
| Hispanic | 190 (7.2) | 794 (11.8) | 154 (8.9) | 789 (11.0) | ||
| Other | 41 (1.6) | 134 (2.0) | 29 (1.7) | 135 (1.9) | ||
| Education, n (%) | <0.001 | 0.003 | ||||
| <High school | 294 (11.1) | 582 (8.7) | 190 (11.0) | 646 (9.0) | ||
| High school diploma | 457 (17.3) | 1063 (15.9) | 301 (17.4) | 1161 (16.2) | ||
| Post high school | 902 (34.1) | 2411 (36.0) | 629 (36.4) | 2542 (35.4) | ||
| College degree | 992 (37.5) | 2644 (39.5) | 610 (35.3) | 2833 (39.5) | ||
| Smoking status, n (%) | <0.001 | 0.02 | ||||
| Never smoker | 1206 (45.6) | 2917 (43.6) | 710 (41.1) | 3206 (44.7) | ||
| Former smoker | 1217 (46.0) | 2756 (41.2) | 786 (45.5) | 3011 (42.0) | ||
| Current smoker | 221 (8.4) | 1019 (15.2) | 232 (13.4) | 958 (13.4) | ||
| Alcohol, n (%) | <0.001 | 0.02 | ||||
| Heavy drinker | 226 (9.0) | 865 (13.5) | 184 (11.2) | 854 (12.5) | ||
| Light drinker | 538 (21.4) | 1382 (21.6) | 320 (19.4) | 1509 (22.1) | ||
| Moderate drinker | 416 (16.6) | 1332 (20.9) | 332 (20.2) | 1325 (19.4) | ||
| Nondrinker | 1331 (53.0) | 2810 (43.9) | 811 (49.2) | 3150 (46.1) | ||
| History of CVD, n (%) | 644 (24.4) | 1233 (18.4) | <0.001 | 423 (24.5) | 1381 (19.2) | <0.001 |
| ACE inhibitor use, n (%) | 1066 (40.3) | 2390 (35.6) | <0.001 | 651 (37.6) | 2650 (36.9) | 0.57 |
| Angiotensin receptor blocker use, n (%) | 603 (22.8) | 1382 (20.6) | 0.02 | 376 (21.7) | 1501 (20.9) | 0.45 |
| Systolic BP, mean±SD, mm Hg | 139.2±16.1 | 139.9±15.4 | 0.04 | 143.7±16.9 | 138.7±15.1 | <0.001 |
| Diastolic BP, mean±SD, mm Hg | 75.0±12.2 | 79.4±11.6 | <0.001 | 78.5±13.4 | 78.0±11.6 | 0.16 |
| Body mass index, mean±SD, kg/m2 | 29.4±5.8 | 30.0±5.8 | <0.001 | 29.7±6.1 | 29.9±5.7 | 0.28 |
| Medical Comorbidity Index ≥5, n (%)a | 1404 (53.2) | 2409 (36.0) | <0.001 | 861 (49.9) | 2795 (38.9) | <0.001 |
| Mental Comorbidity Index ≥2, n (%)a | 214 (8.1) | 682 (10.2) | 0.002 | 154 (8.9) | 702 (9.8) | 0.27 |
| eGFR, mean±SD, ml/min per 1.73 m2 | 47.9±9.5 | 81.2±15.5 | <0.001 | 63.1±22.8 | 74.5±18.8 | <0.001 |
| UACR, median (interquartile range), mg/g | 13.3 (6.4–43.1) | 8.6 (5.5–17.1) | <0.001 | 66.7 (42.1–162.1) | 7.7 (5.11–12.6) | <0.001 |
| Framingham 10-yr cardiovascular risk score, mean±SD, % | 27.1±14.3 | 23.9±11.7 | <0.001 | 28.6±14.2 | 24.1±12.0 | <0.001 |
| Montreal Cognitive Assessment, median (interquartile range)b | 23 (20–26) | 24 (21–26) | <0.001 | 23 (20–25) | 24 (21–26) | <0.001 |
P values on the basis of chi-square tests for categorical variables and t tests for continuous variables, with the exception of variables presented as median (interquartile range), which were tested with Wilcoxon rank sum tests. ACE, angiotensin-converting enzyme.
Scores on the Medical Comorbidity Index range from 0 to 30, with higher scores indicating more coexisting medical conditions. Scores on the Mental Comorbidity Index range from 0 to 6, with higher scores indicating more coexisting mental health conditions.
Scores for the Montreal Cognitive Assessment range from 0 to 30. Higher scores denote better cognitive function.
A total of 8563 (91.5%) participants completed at least one cognitive assessment during follow-up. As compared with participants who did complete a cognitive assessment during follow-up, those who did not complete a cognitive assessment had a similar prevalence of eGFR<60 ml/min per 1.73 m2 and were more likely to have UACR>30 mg/g at baseline. Over a median follow-up of 5.1 years, probable dementia occurred in 325 (3.8%) and MCI in 640 (7.6%) participants. The median number of eGFR measurements during follow-up was 10 (interquartile range, 8–11) and the median number of UACR measurements during follow-up was 5 (interquartile range, 4–6). Figure 1 depicts kidney events and cognitive follow-up, stratified by treatment arm and baseline eGFR.
Figure 1.
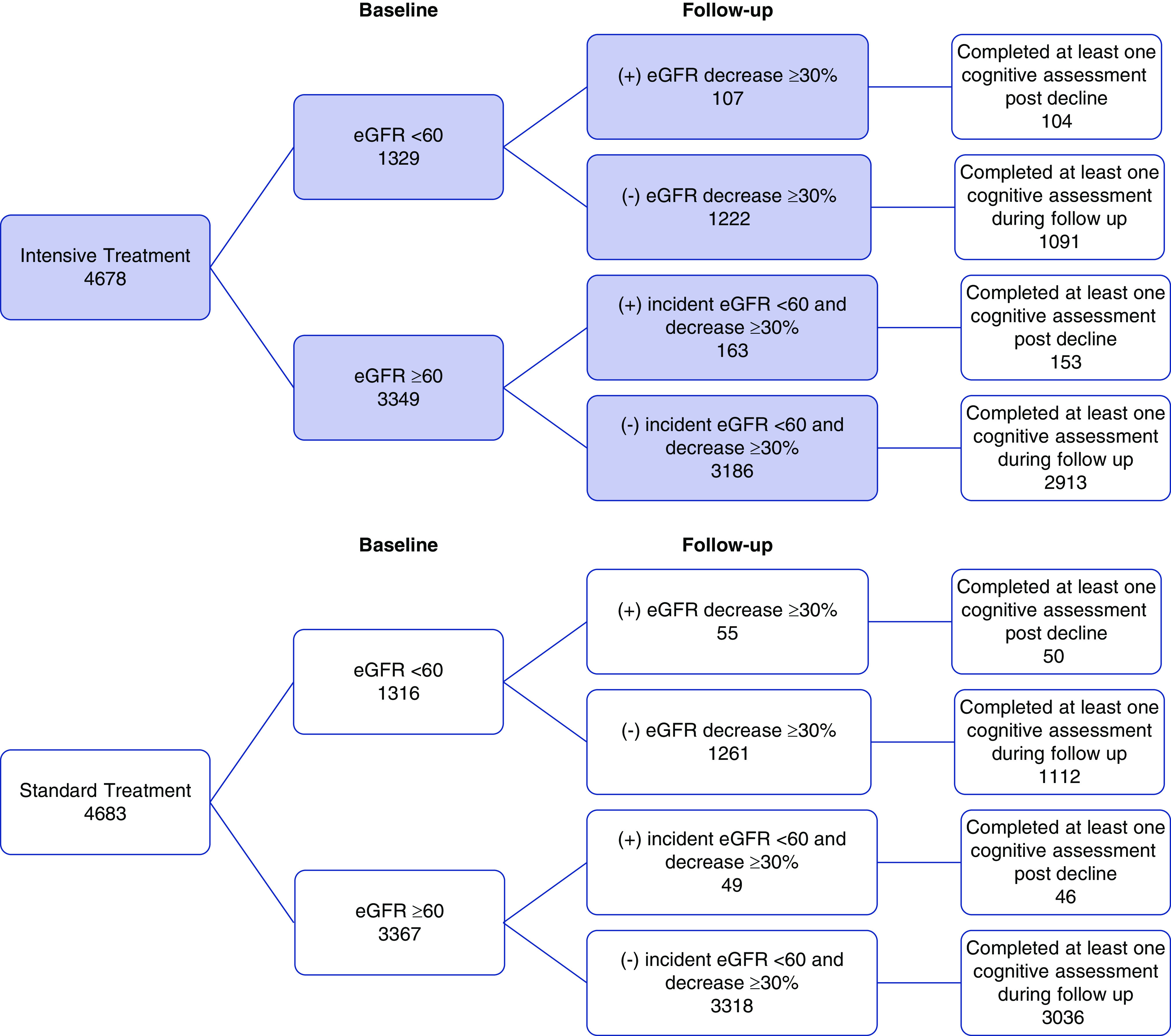
Flow diagram of participants, stratified by treatment arm and baseline eGFR. Decline in eGFR ≥30% and incident eGFR <60 ml/min per 1.73 m2 occurred more frequently in the intensive versus standard treatment group.
Association of Baseline eGFR and UACR with Dementia and MCI
In adjusted analyses, participants with baseline eGFR <60 ml/min per 1.73 m2 were not at increased risk for probable dementia, MCI, or the composite outcome compared with participants with eGFR≥60 ml/min per 1.73 m2 (Table 2). There was a modestly increased risk for MCI and the composite of probable dementia or MCI among participants with baseline eGFR <45 ml/min per 1.73 m2 but not among those with eGFR 45–59 ml/min per 1.73 m2 (Supplemental Table 1). Similarly, participants with baseline UACR ≥30 mg/g were not at increased risk for dementia, MCI, or the composite outcome, compared with participants with UACR<30 mg/g (Table 2). The association between UACR≥300 mg/g and dementia risk (HR, 2.38; 95% confidence interval [95% CI], 0.84 to 6.71) or MCI risk was not statistically significant (Supplemental Table 1). In sensitivity analyses accounting for the competing risk of death, the findings were similar (Supplemental Table 2).
Table 2.
Association of baseline eGFR and UACR with the risk for cognitive impairment
| Baseline Kidney Markers | Probable Dementia | MCI | Probable Dementia or MCI | |||
|---|---|---|---|---|---|---|
| Cases per 1000 PY | Adjusted HR (95% CI)a | Cases per 1000 PY | Adjusted HR (95% CI)a | Cases per 1000 PY | Adjusted HR (95% CI)a | |
| eGFR, ml/min per 1.73 m2 | ||||||
| eGFR<60 | ||||||
| Yes (n=2385) | 13.0 | 1.04 (0.80 to 1.36) | 21.9 | 0.98 (0.80 to 1.18) | 31.1 | 1.02 (0.86 to 1.20) |
| No (n=6178) | 12.1 | Referent | 14.5 | Referent | 18.9 | Referent |
| UACR, mg/g | ||||||
| UACR≥30 | ||||||
| Yes (n=1539) | 12.4 | 1.24 (0.93 to 1.66) | 22.3 | 1.11 (0.90 to 1.38) | 31.4 | 1.05 (0.88 to 1.26) |
| No (n=6627) | 7.1 | Referent | 15.3 | Referent | 20.3 | Referent |
PY, person-years.
Adjusted HR on the basis of the Cox proportional hazards regression model with the baseline hazard function stratified by clinic site. Model covariates include treatment assignment, age, sex, race, education, systolic BP, diastolic BP, angiotensin-converting enzyme inhibitor use, angiotensin receptor blocker use, smoking status, alcohol use, body mass index, Montreal Cognitive Assessment score, Framingham 10-year cardiovascular risk score, Medical and Mental Comorbidity Index, and UACR (in eGFR model) or eGFR (in UACR model).
Association of Longitudinal Change in eGFR and UACR with Dementia and MCI
Among all participants, decline in eGFR ≥30% from baseline occurred in 465 (5.0%) participants. In a time-varying model adjusted for baseline covariates and treatment assignment, decline in eGFR ≥30% was associated with increased risk for probable dementia (HR, 1.76; 95% CI, 1.03 to 2.77) (Table 3). Among participants with eGFR≥60 ml/min per 1.73 m2 at baseline, incident eGFR <60 ml/min per 1.73 m2 occurred in 212 (3.2%). Although there was a nonsignificant increased risk for dementia, there was a significant increased risk for MCI (HR, 2.10; 95% CI, 1.19 to 3.70), and the composite outcome (HR, 2.14; 95% CI, 1.35 to 3.40) (Table 3). There was no significant association between incident UACR ≥30 mg/g with dementia or MCI. In sensitivity analyses accounting for the competing risk of death, the findings were similar (Supplemental Table 3).
Table 3.
Association of postrandomization declines in eGFR and increases in UACR with the risk for subsequent cognitive impairment during follow-up
| Kidney Marker | N | HR (95% CI) | ||
|---|---|---|---|---|
| Probable Dementia | MCI | Probable Dementia or MCI | ||
| eGFR, ml/min per 1.73 m2 | ||||
| Decline in eGFR ≥30% | ||||
| Yes | 465 | 1.76 (1.03 to 2.77) | 0.99 (0.61 to 1.60) | 1.19 (0.83 to 1.70) |
| No | 8896 | Referent | Referent | Referent |
| Incident CKD | ||||
| Yes | 212 | 1.70 (0.89 to 3.25) | 2.10 (1.19 to 3.70) | 2.14 (1.35 to 3.40) |
| No | 6450 | Referent | Referent | Referent |
| UACR, mg/g | ||||
| Incident UACR ≥30 | ||||
| Yes | 1837 | 0.90 (0.68 to 1.19) | 0.91 (0.70 to 1.18) | 0.93 (0.76 to 1.15) |
| No | 5053 | Referent | Referent | Referent |
Models on the basis of Cox proportional hazards regression with the baseline hazard function stratified by clinic site, including treatment group, age, sex, race, education, systolic BP, diastolic BP, angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, smoking status, alcohol use, body mass index, Montreal Cognitive Assessment score, Framingham 10-year cardiovascular risk score, Medical and Mental Comorbidity Index, and baseline UACR (in eGFR model) or baseline eGFR (in UACR model).
Kidney Markers, Intensive Hypertension Treatment, and Dementia Risk
As previously reported, intensive hypertension treatment, as compared with standard treatment, resulted in a nonsignificant decrease in the incidence of probable dementia, and a significant decrease in the incidence of MCI and the composite outcome.12 Baseline eGFR did not modify the effect of intensive treatment on the incidence of probable dementia (Table 4). There was a significant interaction between intensive treatment and eGFR on MCI incidence (P value for interaction =0.04). Intensive treatment, as compared with standard treatment, was associated with a lower incidence of MCI among participants with eGFR≥60 ml/min per 1.73 m2 (HR, 0.71; 95% CI, 0.58 to 0.87), but not among participants with eGFR<60 ml/min per 1.73 m2 (HR, 1.01; 95% CI, 0.77 to 1.31). In sensitivity analyses accounting for the competing risk of death, the findings were similar (Supplemental Table 4). Continuous analyses of eGFR were supportive of this result, with HR estimates indicating benefit of intensive treatment for participants with baseline eGFR ≥60 ml/min per 1.73 m2, and conversely no indication of benefit for participants with eGFR <60 ml/min per 1.73 m2 (Figure 2).
Table 4.
Effect of intensive BP target on cognitive impairment as a function of baseline eGFR and UACR
| Outcome | Intensive Treatment | Standard Treatment | Intensive versus Standard | Interaction P Value |
|---|---|---|---|---|
| No. of Cases/Cases per 1000 PY | No. of Cases/Cases per 1000 PY | HR (95% CI)a | ||
| eGFR, ml/min per 1.73 m2 | ||||
| Probable dementia | 0.95 | |||
| eGFR ≥60 | 81/5.4 | 100/6.7 | 0.81 (0.61 to 1.10) | |
| eGFR <60 | 68/12.0 | 76/14.1 | 0.79 (0.56 to 1.11) | |
| MCI | 0.04 | |||
| eGFR ≥60 | 170/11.8 | 244/17.1 | 0.71 (0.58 to 0.86) | |
| eGFR <60 | 117/22.1 | 109/21.7 | 1.00 (0.77 to 1.31) | |
| Probable dementia or MCI | 0.12 | |||
| eGFR ≥60 | 237/16.4 | 307/21.4 | 0.77 (0.65 to 0.92) | |
| eGFR <60 | 165/30.6 | 162/31.6 | 0.96 (0.77 to 1.20) | |
| UACR, mg/g | ||||
| Probable dementia | 0.27 | |||
| UACR <30 | 101/6.3 | 125/7.8 | 0.78 (0.60 to 1.02) | |
| UACR ≥30 | 44/12.3 | 43/12.5 | 1.08 (0.69 to 1.68) | |
| MCI | 0.43 | |||
| UACR <30 | 208/13.5 | 259/17.1 | 0.80 (0.67 to 0.96) | |
| UACR ≥30 | 72/21.5 | 74/23.1 | 0.89 (0.63 to 1.25) | |
| Probable dementia or MCI | 0.10 | |||
| UACR <30 | 283/18.2 | 342/22.5 | 0.82 (0.70 to 0.96) | |
| UACR ≥30 | 108/31.7 | 101/31.0 | 1.04 (0.78 to 1.38) |
PY person-years.
Intensive treatment group versus standard treatment group on the basis of Cox proportional hazards regression.
Figure 2.
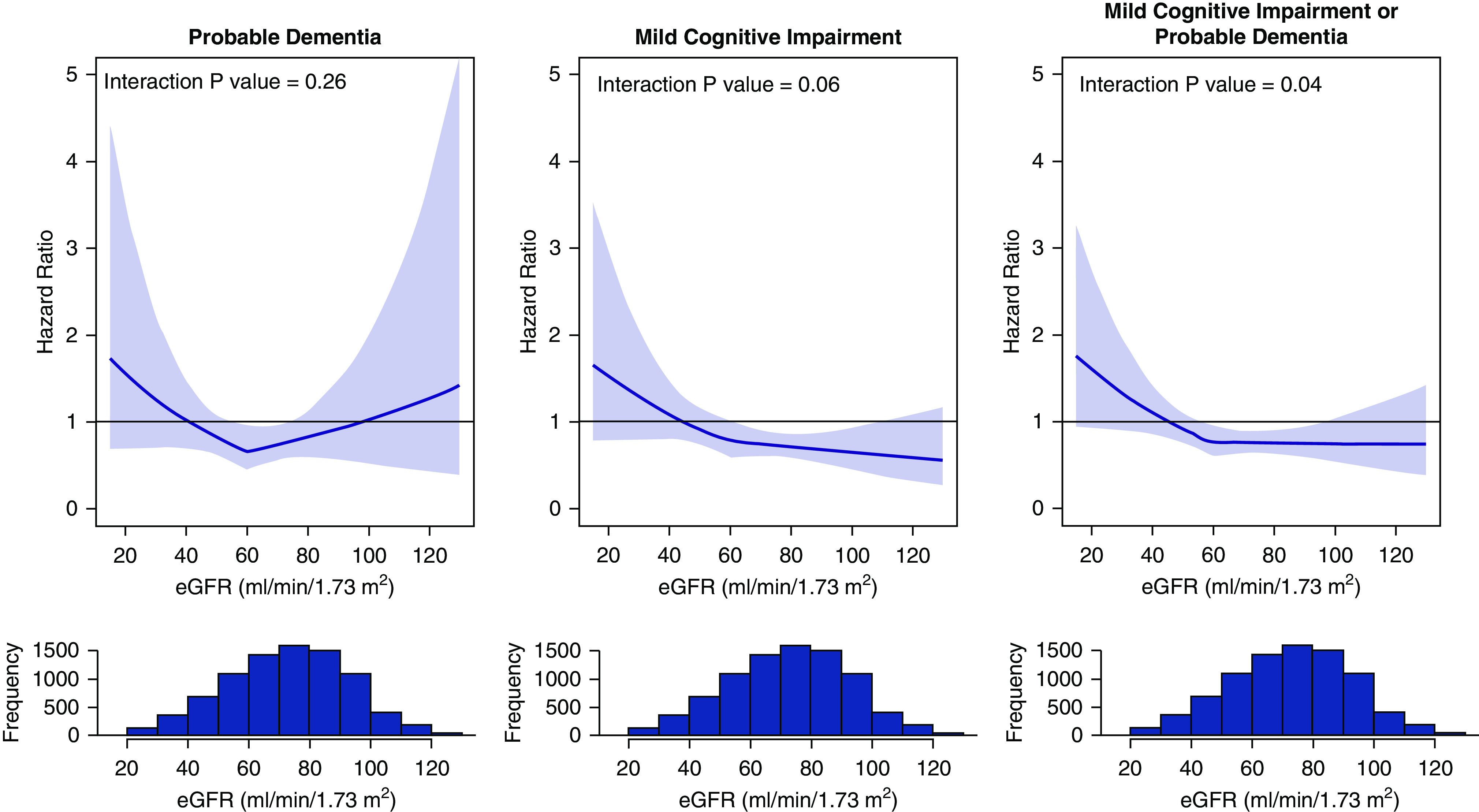
Effect of intensive BP control versus standard treatment on probable dementia and mild cognitive impairment as a function of baseline eGFR. Hazard ratio estimates indicate no benefit of intensive treatment on cognitive outcomes for participants with eGFR <60 ml/min per 1.73 m2. Bold lines denote hazard ratio comparing intensive treatment to standard treatment on the basis of a Cox proportional hazards model with an interaction between eGFR and treatment group, with eGFR modeled continuously with a breakpoint at 60 ml/min/1.73 m2. Shaded areas denote 95% point-wise confidence intervals. Histograms reflect baseline distribution of eGFR pooled across treatment groups.
Baseline UACR did not modify the effect of intensive treatment on incidence of probable dementia and MCI. When the treatment interaction with UACR was modeled as a linear function with a break point at 30 mg/g, the HR suggested a benefit of intensive treatment below this level of UACR, and no benefit above this level (Supplemental Figure 1).
Although decline in eGFR ≥30% and incident eGFR <60 ml/min per 1.73 m2 occurred more frequently in the intensive treatment group compared with the standard treatment group, they did not modify the effect of intensive treatment on dementia or MCI (P values for interaction >0.10). Furthermore, adjustment for either decline in eGFR >30% or incident eGFR <60 ml/min per 1.73 m2 did not materially change the significant association between intensive treatment with the composite outcome (Table 5). For example, the HR for the overall treatment effect was 0.85 (95% CI, 0.74 to 0.97), and after adjustment for eGFR decline >30%, the HR was 0.88 (95% CI, 0.77 to 1.01).
Table 5.
Association of intensive BP target on cognitive impairment in baseline model, and with adjustment for baseline eGFR or postrandomization eGFR declines
| eGFR, ml/min per 1.73 m2 | HR (95% CI) for Intensive versus Standard | ||
|---|---|---|---|
| Probable Dementia | MCI | Probable Dementia or MCI | |
| Original | 0.83 (0.67 to 1.04) | 0.81 (0.69 to 0.94) | 0.85 (0.74 to 0.97) |
| Baseline eGFR <60 model | 0.82 (0.66 to 1.03) | 0.80 (0.69 to 0.94) | 0.84 (0.74 to 0.96) |
| Decline in eGFR ≥30% model | 0.85 (0.69 to 1.06) | 0.84 (0.72 to 0.99) | 0.88 (0.77 to 1.01) |
| Incident eGFR <60 model | 0.84 (0.63 to 1.12) | 0.73 (0.60 to 0.89) | 0.80 (0.67 to 0.94) |
Discussion
In a large clinical trial of hypertension treatment intensity, we found that ≥30% decline in baseline eGFR and incident eGFR <60 ml/min per 1.73 m2 were associated with an increased incidence of probable dementia and MCI. Although these kidney events occurred more frequently in the intensive treatment group, there was no evidence that they modified or attenuated the effect of intensive treatment on dementia and MCI incidence. In contrast, baseline and incident UACR ≥30 mg/g were not associated with incidence of probable dementia or MCI, nor did UACR modify the effect of intensive treatment on these outcomes. These findings are consistent with the hypothesis that declining kidney function and declining cognitive function share a common pathogenesis independent of BP control, and imply that large declines in kidney function may identify a population at higher risk for adverse cognitive outcomes.
Previous studies have suggested a link between CKD and cognitive impairment, but few have evaluated the association between kidney markers and the clinically meaningful end-points of dementia and MCI.1,9,18 These findings from SPRINT confirm and extend results from two population-based cohort studies that reported an increased risk for incident dementia associated with rapid eGFR decline and eGFR variability, but not with baseline eGFR or UACR.9,18 In our study, the stronger association between longitudinal as compared with baseline measures of eGFR with incident dementia is most likely because of imprecision of a single creatinine-based measure of GFR and the low baseline prevalence of advanced kidney disease in SPRINT.
Our study adds novel information about the interactions between kidney disease and hypertension treatment on cognitive outcomes. Intensive treatment of hypertension increases the risk for eGFR decline and incident CKD.13,19,20 The clinical significance of changes in kidney function that occur in the context of hypertension treatment is controversial. Kidney function decline after the intensification of hypertension treatment is mild in the majority of cases and not associated with downstream cardiovascular risk,21,22 which has led to the assertion that these changes reflect reversible hemodynamic effects rather than permanent ischemic damage. A post hoc analysis of two CKD hypertension trials found that ≥20% decline in eGFR was associated with a higher risk for CKD progression in both intensive and standard treatment groups, whereas smaller declines in eGFR were associated with CKD progression only in the standard treatment group.23 A recent post hoc analysis of SPRINT found that early eGFR decline did not mediate or modify the effects of intensive treatment on mortality or cardiovascular events.24
In our study, baseline eGFR did not modify the effect of intensive treatment on probable dementia, but there was a statistically significant interaction between baseline eGFR and MCI. Specifically, the beneficial effect of intensive treatment on MCI was not observed among participants with a baseline eGFR <60 ml/min per 1.73 m2. This result could be because of chance or it may reflect lower power owing to lower dementia event rates. Consistent with the possibility this observation was because of chance, there was no evidence that eGFR decline, whether defined as ≥30% decline in eGFR or incident eGFR <60 ml/min per 1.73 m2, modified the effect of hypertension treatment. Furthermore, the overall treatment effect HRs did not materially differ after accounting for eGFR decline, indicating that declining kidney function did not attenuate the intervention’s effects on cognitive outcomes. On the other hand, exploratory analyses modeling the treatment interaction as a linear function of eGFR suggested the possibility of harm from intensive BP treatment at low levels of eGFR. However, SPRINT included very few of these individuals with a small number of events. As such, this finding should be viewed as hypothesis generating and interpreted cautiously.
The brain and kidney are both high-flow low-resistance end organs. These characteristics make them uniquely susceptible to excess pressure pulsatility if autoregulatory capacity is impaired.25,26 Apart from hemodynamic effects of hypertension treatment, shared vascular risk factors, such as arterial stiffness, vascular calcification, and endothelial dysfunction, may disrupt small vessel autoregulation. Additional studies in SPRINT evaluating brain structure and perfusion should provide insight into the mechanisms that link eGFR decline and adverse cognitive outcomes.27
SPRINT was a large, randomized, clinical trial with long-term follow-up, repeated measurements of eGFR and UACR, and adjudicated outcome assessments for dementia and MCI. We used predefined kidney end points in SPRINT as our main longitudinal exposures, supplemented with analysis of kidney function as a continuous variable to evaluate heterogeneity of treatment effect across the continuum of eGFR and UACR. Although there are several strengths of our study, there are also some important limitations. First, a small proportion of participants had an eGFR<45 ml/min per 1.73 m2 or high levels of albuminuria, limiting power for subgroup analyses. Second, statistical power to detect treatment effect heterogeneity was likely negatively affected by the early termination of the SPRINT intervention and the typical late onset of dementia, which in the prevention context, usually necessitates follow-up periods of >5 years.28 Although the analyses of subgroup effects by baseline GFR and UACR preserves the randomized treatment assignment in SPRINT, the analyses of longitudinal changes in GFR and UACR does not, and is susceptible to confounding similar to observational analyses. Although we adjusted for a large number of potential confounders, there may be unmeasured residual confounding present. Finally, clinical trial participants may not be reflective of clinical populations with CKD.
In conclusion, declining eGFR may be a marker for those at higher risk for dementia or MCI, but it did not appear to modify or attenuate the effects of intensive hypertension treatment.
Disclosures
S. Oparil reports grants and nonfinancial support from National Institutes of Health (NIH)/National Heart, Lung and Blood Institute, during the conduct of the study; and outside the submitted work, has received personal fees from 98point6, Inc, Actelion Clinical Research Inc, CinCor, Novo Nordisk, Pfizer, and Rox Medical, research/grant funding from Bayer, George Clinical Pty Ltd/Actelion/Idorsia Pharmaceuticals, Idorsia Pharmaceuticals, and Novartis, and currently serves as Editor-in-Chief for Current Hypertension Reports (publisher: Springer); and receives an annual stipend of $5000 (from Springer). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the US Department of Veterans Affairs, or the US Government. For a full list of contributors to SPRINT, please see the supplementary acknowledgement list: https://www.sprinttrial.org/public/dspScience.cfm. All remaining authors have nothing to disclose.
Funding
This study was supported by National Institutes of Health (NIH) grants R01DK092241 (to M. Kurella Tamura) and R01AG055606 (to PI: Dr. Reboussin), as well as funding from the Alzheimer’s Association (to J.D. Williamson). The Systolic Blood Pressure Intervention Trial is funded with Federal funds from the NIH, including the National Heart, Lung, and Blood Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Aging, and the National Institute of Neurological Disorders and Stroke, under contract numbers HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, and HHSN268200900049C, and interagency agreement number A-HL-13-002-001.
Supplementary Material
Acknowledgments
This study was also supported in part by resources and use of facilities through the US Department of Veterans Affairs. The SPRINT investigators acknowledge the contribution of study medications (azilsartan and azilsartan combined with chlorthalidone) from Takeda Pharmaceuticals International, Inc.
All components of the SPRINT study protocol were designed and implemented by the investigators. The investigative team collected, analyzed, and interpreted the data. All aspects of manuscript writing and revision were carried out by the coauthors.
The authors thank the participants of the SPRINT study for their important contributions. For a full list of contributors to SPRINT, please visit www.sprinttrial.org.
Dr. Manjula Kurella Tamura reports grants from National Institutes of Health (NIH), during the conduct of the study. Ms. Sarah Gaussoin reports grants from NIH, during the conduct of the study. Dr. Nicholas M. Pajewski reports grants from National Institute on Aging and Alzheimer’s Association, during the conduct of the study. Dr. Gorden Chelune reports grants from NIH during the conduct of the study. Dr. Barry I. Freedman reports grants from NIH, during the conduct of the study. Dr. Anthony Killeen reports grants from NIH/NIDDK, during the conduct of the study. Dr. Mark Supiano reports grants from NIH, during the conduct of the study. Dr. Daniel E. Weiner reports grants from National Institutes of Health, during the conduct of the study. Dr. Jeff D. Williamson reports grants from National Institutes of Health, grants from Biogen, and grants from Alzheimer's Association, outside the submitted work.
We also acknowledge support from the following Clinical and Translational Science Awards funded by National Center for Advancing Translational Science: Case Western Reserve University, UL1TR000439; OSU, UL1RR025755; U Penn, UL1RR024134 and UL1TR000003; Boston, UL1RR025771; Stanford, UL1TR000093; Tufts, UL1RR025752, UL1TR000073, and UL1TR001064; University of Illinois, UL1TR000050; University of Pittsburgh, UL1TR000005; UT Southwestern, 9U54TR000017-06; University of Utah, UL1TR000105-05; Vanderbilt University, UL1TR000445; George Washington University, UL1TR000075; University of CA, Davis, UL1TR000002; University of Florida, UL1TR000064; University of Michigan, UL1TR000433; Tulane University, P30GM103337 COBRE Award NIGMS; Wake Forest University, UL1TR001420.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Contributor Information
Collaborators: Manjula Kurella Tamura, Sarah A. Gaussoin, Nicholas M. Pajewski, Gordon J. Chelune, Barry I. Freedman, Tanya R. Gure, William E. Haley, Anthony A. Killeen, Suzanne Oparil, Stephen R. Rapp, Dena Rifkin, Mark Supiano, Jeff D. Williamson, and Daniel E. Weiner
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020010038/-/DCSupplemental.
Supplemental Table 1. Adjusted association of baseline eGFR and UACR with risk for dementia and MCI.
Supplemental Table 2. Association of baseline eGFR and UACR with the risk for cognitive impairment accounting for the competing risk of death.
Supplemental Table 3. Association of postrandomization declines in eGFR and increases in UACR with the risk for subsequent cognitive impairment during follow-up, accounting for the competing risk of death.
Supplemental Table 4. Effect of intensive BP control on cognitive impairment, accounting for the competing risk of death, as a function of baseline eGFR and UACR.
Supplemental Figure 1. Effect of intensive BP control on probable dementia and MCI as a function of baseline UACR.
Supplemental Appendix. Full list of contributors to SPRINT.
References
- 1.Seliger SL, Siscovick DS, Stehman-Breen CO, Gillen DL, Fitzpatrick A, Bleyer A, et al.: Moderate renal impairment and risk of dementia among older adults: The cardiovascular health cognition study. J Am Soc Nephrol 15: 1904–1911, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Kurella M, Chertow GM, Fried LF, Cummings SR, Harris T, Simonsick E, et al.: Chronic kidney disease and cognitive impairment in the elderly: The health, aging, and body composition study. J Am Soc Nephrol 16: 2127–2133, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Fryar CDOY, Hales CM, Zhang G, Kruszon-Moran D: Hypertension prevalence and control among adults: United States, 2015-2016. NCHS Data Brief 289: 1–8, 2017 [PubMed] [Google Scholar]
- 4.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, et al.: 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: Executive summary: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines [published correction appears in Hypertension 72: e33, 2018]. Hypertension 71: 1269–1324, 2018. [DOI] [PubMed] [Google Scholar]
- 5.Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, et al.: Modification of Diet in Renal Disease Study Group : The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Engl J Med 330: 877–884, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Appel LJ, Wright JT Jr., Greene T, Agodoa LY, Astor BC, Bakris GL, et al.: AASK Collaborative Research Group : Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med 363: 918–929, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacEwen C, Sutherland S, Daly J, Pugh C, Tarassenko L: Relationship between hypotension and cerebral ischemia during hemodialysis. J Am Soc Nephrol 28: 2511–2520, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eldehni MT, Odudu A, McIntyre CW: Randomized clinical trial of dialysate cooling and effects on brain white matter. J Am Soc Nephrol 26: 957–965, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helmer C, Stengel B, Metzger M, Froissart M, Massy ZA, Tzourio C, et al.: Chronic kidney disease, cognitive decline, and incident dementia: The 3C study. Neurology 77: 2043–2051, 2011. [DOI] [PubMed] [Google Scholar]
- 10.Weiner DE, Gaussoin SA, Nord J, Auchus AP, Chelune GJ, Chonchol M, et al.: SPRINT Study Research Group : Cognitive function and kidney disease: Baseline data from the systolic blood pressure intervention trial (SPRINT). Am J Kidney Dis 70: 357–367, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Georgakis MK, Chatzopoulou D, Tsivgoulis G, Petridou ET: Albuminuria and cerebral small vessel disease: A systematic review and meta-analysis. J Am Geriatr Soc 66: 509–517, 2018. [DOI] [PubMed] [Google Scholar]
- 12.Williamson JD, Pajewski NM, Auchus AP, Bryan RN, Chelune G, Cheung AK, et al.: SPRINT MIND Investigators for the SPRINT Research Group : Effect of intensive vs standard blood pressure control on probable dementia: A randomized clinical trial. JAMA 321: 553–561, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright JT Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, et al.: SPRINT Research Group : A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 373: 2103–2116, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, et al.: SPRINT Study Research Group : The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: The systolic blood pressure intervention trial (SPRINT). Clin Trials 11: 532–546, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Selim AJ, Fincke G, Ren XS, Lee A, Rogers WH, Miller DR, et al.: Comorbidity assessments based on patient report: Results from the Veterans Health Study. J Ambul Care Manage 27: 281–295, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Welsh KA: Detection of early dementia in the elderly. Exp Aging Res 17: 101, 1991. [PubMed] [Google Scholar]
- 18.O’Hare AM, Walker R, Haneuse S, Crane PK, McCormick WC, Bowen JD, et al.: Relationship between longitudinal measures of renal function and onset of dementia in a community cohort of older adults. J Am Geriatr Soc 60: 2215–2222, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cushman WC, Evans GW, Byington RP, Goff DC Jr., Grimm RH Jr., Cutler JA, et al.: ACCORD Study Group : Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 362: 1575–1585, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peralta CA, McClure LA, Scherzer R, Odden MC, White CL, Shlipak M, et al.: Effect of intensive versus usual blood pressure control on kidney function among individuals with prior lacunar stroke: A post hoc analysis of the secondary prevention of small subcortical strokes (SPS3) randomized trial. Circulation 133: 584–591, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rocco MV, Sink KM, Lovato LC, Wolfgram DF, Wiegmann TB, Wall BM, et al.: SPRINT Research Group : Effects of intensive blood pressure treatment on acute kidney injury events in the systolic blood pressure intervention trial (SPRINT). Am J Kidney Dis 71: 352–361, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ku E, Glidden DV, Johansen KL, Sarnak M, Tighiouart H, Grimes B, et al.: Association between strict blood pressure control during chronic kidney disease and lower mortality after onset of end-stage renal disease. Kidney Int 87: 1055–1060, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ku E, Bakris G, Johansen KL, Lin F, Sarnak MJ, Campese VM, et al.: Acute declines in renal function during intensive BP lowering: Implications for future ESRD risk. J Am Soc Nephrol 28: 2794–2801, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beddhu S, Shen J, Cheung AK, Kimmel PL, Chertow GM, Wei G, et al.: Implications of early decline in eGFR due to intensive BP control for cardiovascular outcomes in SPRINT. J Am Soc Nephrol 30: 1523–1533, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell GF: Effects of central arterial aging on the structure and function of the peripheral vasculature: Implications for end-organ damage. J Appl Physiol (1985) 105: 1652–1660, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seliger SL, Longstreth WT Jr.: Lessons about brain vascular disease from another pulsating organ, the kidney. Stroke 39: 5–6, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Nasrallah IM, Pajewski NM, Auchus AP, Chelune G, Cheung AK, Cleveland ML, et al.: SPRINT MIND Investigators for the SPRINT Research Group : Association of intensive vs standard blood pressure control with cerebral white matter lesions. JAMA 322: 524–534, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whiteley WN, Anand S, Bangdiwala SI, Bosch J, Canavan M, Chertkow H, et al.: Are large simple trials for dementia prevention possible? Age Ageing 49: 154–160, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.