Figure 5: Silica nanoparticles increased permeability by binding cell surface integrins and inducing tight junction rearrangement.
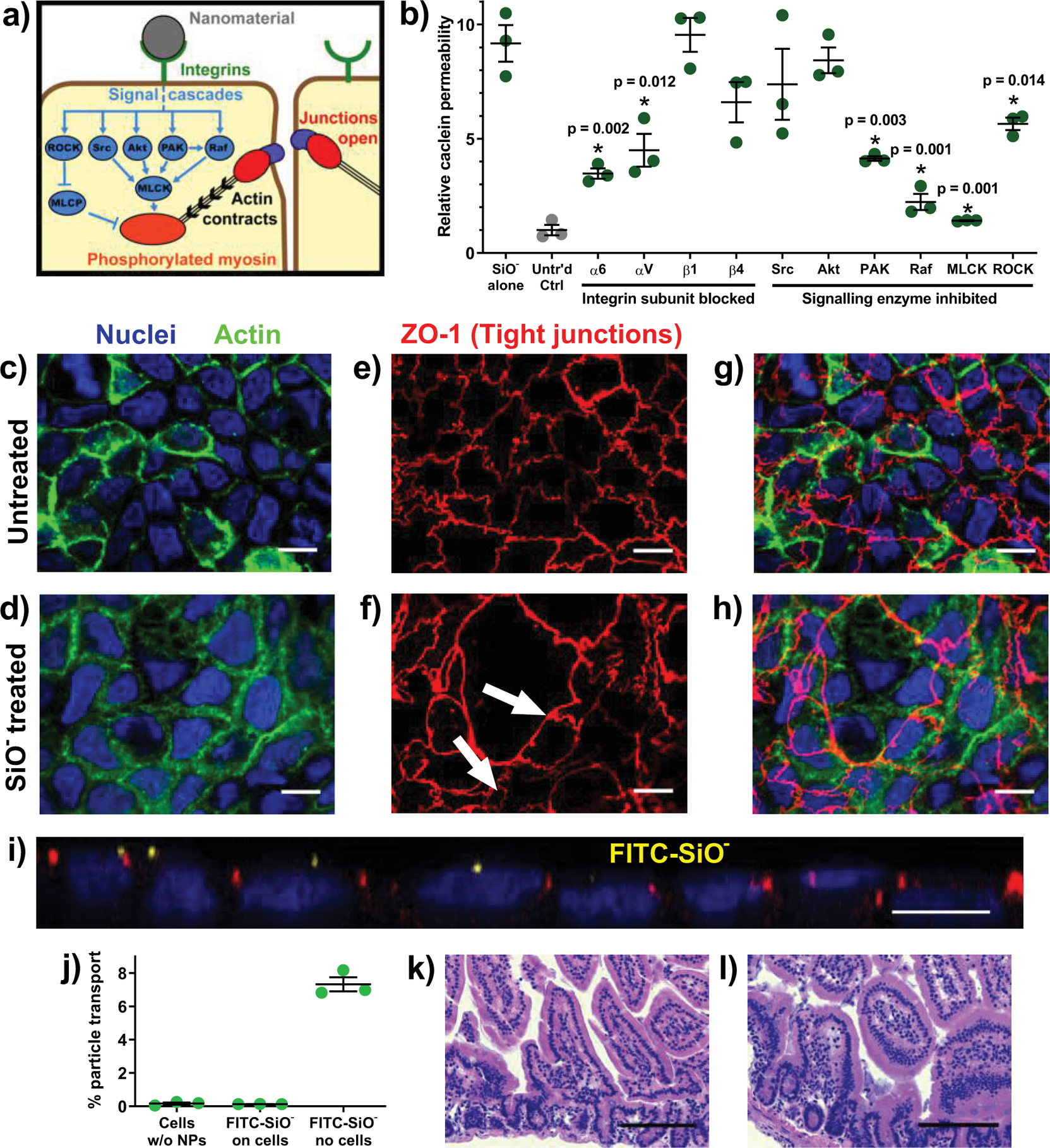
(a) There are several pathways through which integrin activation and myosin light chain phosphorylation are linked to intestinal permeability. (b) The permeation enhancing effect of silica nanoparticles in vitro was reduced by blocking particle binding to integrin α subunits or by inhibiting a subset of intracellular signalling cascade proteins. (c, d) In Caco-2 monolayers imaged at 63x magnification, actin (green) and nuclei (blue) did not rearrange in response to particle treatment. (e) The tight junction protein ZO-1 (red) localised around the perimeter of each untreated cell while (f) silica particle treatment induced ZO-1 rearrangement, creating clusters of cells between which no ZO-1 was present (white arrows). (g) Overlaid images show that the general 1:1 ratio of tight junctions to nuclei in the untreated sample was not maintained in (h) the nanoparticle treated cells, where a single tight junctional boundary encompassed several nuclei. Monolayer images displayed here are a representative sample of over twenty images captured from each treatment. (i) A side view confocal image of a nanoparticle-treated monolayer confirmed that nanoparticles (yellow) localised on top of the cells but did not permeate the tight junctions. (j) FITC-labelled silica nanoparticles did not cross in vitro intestinal barriers. Particles diffused into the basolateral chamber when no epithelial cells were present. (k) Histological analysis of untreated and (l) particle treated mice showed no inflammation and no change in epithelial architecture. Statistics bars display arithmetic mean and s.e.m. for biological replicates (n = 3). * p < 0.05 by one-tailed t-test. White scale bars = 10 μm. Black scale bars = 100 μm.
