Figure 4. GCN5L1 modulates the acetylation of mitochondrial electron transport chain proteins.
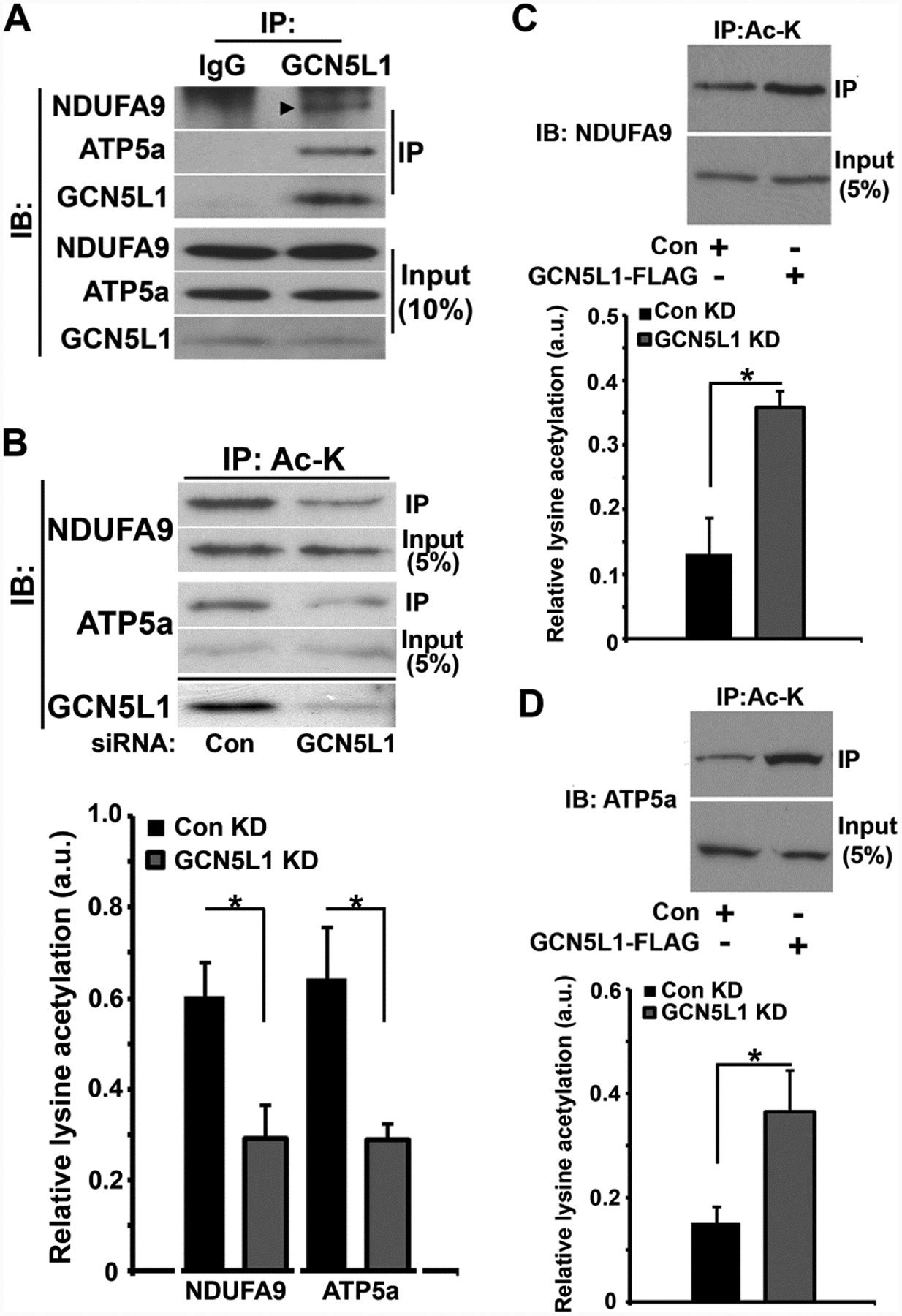
(A) In vivo interaction of GCN5L1 with the ETC proteins NDUFA9 and ATP5a. The arrow shows the specific band for NDUFA9 relative to the non-specific band seen in the IgG immunoprecipitation control. (B) In vitro immunoprecipitation acetylation assay of endogenous NDUFA9 and ATP5a with accompanying histogram showing the relative levels of the respective protein acetylation (Ac-K) levels in control or GCN5L1-depleted HepG2 cells. (C) In vitro immunoprecipitation acetylation assay using an acetylated-lysine antibody to assay endogenous NDUFA9 acetylation with the accompanying histogram showing relative acetylation in HepG2 cells expressing control or GCN5L1 plasmids . (D) In vitro immunoprecipitation acetylation assay using an acetylated-lysine antibody to assay endogenous ATP5a acetylation with the accompanying histogram showing relative acetylation in HepG2 cells expressing control or GCN5L1 plasmids. n ≥ 3 for all experiments. *p<0.05, compared to respective controls. Results are mean ± s.e.m.
