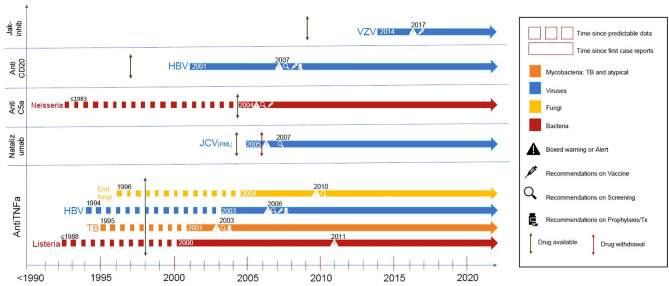
Figure 1.
These are representative infectious complications from major targeted therapies. The dotted line demonstrates the time where there was supportive evidence either from preclinical models, clinical case reports, or evidence from primary immunodeficiency states was apparent. The dotted line becomes solid at about the time of the first case reports of this complication, followed by a green arrow indicating date of drug approval and a red arrow indicating drug withdrawal if such occurred. Additional symbols (see legend) indicate regulatory modifications of labeling or guidelines of care (screening, vaccination, or prophylaxis) when they occurred. References are in the supplement (Supplemental Data 1).