Figure 6.
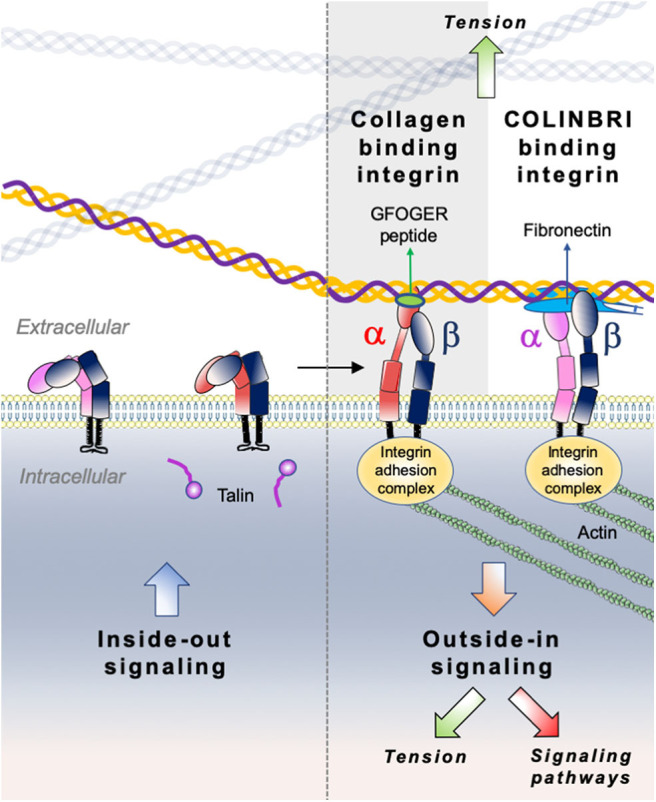
Illustration of direct (collagen-binding integrin-mediated) and indirect (COLINBRI-mediated) integrin heterodimers binding to collagen fibrils. Inactive integrins adopt a compact conformation in which the α- (red/purple) and β-subunit (black) are closely associated. Intracellular signals, culminating in the binding of talin to the β-subunit tail, lead to conformational changes that result in increased affinity for extracellular ligands. The primed integrin binds ligand, which represents the end-point of inside-out signaling. The binding of talin and ligand initiate focal contact formation. As the cytoskeleton matures, tension (green arrows) is generated on the integrin receptor across the cell membrane. The force applied to the integrin strengthens receptor-ligand binding and allows the formation of stable focal adhesions and the initiation of intracellular signaling cascades (red arrow), the end-point of outside-in signaling. In the direct cell-binding mechanism, collagen-binding integrins directly interact with the GFOGER sequence of fibrillar collagen to provide cell adhesion. In the indirect way, cell binding involves COLINBRIs like fibronectin represented here in blue. The COLINBRI molecule is anchored to collagen and provides cell attachment by interaction with the COLINBRI-binding integrins.
