Abstract
Pericytes (PCs) are a type of perivascular cells that surround endothelial cells of small blood vessels. In the brain, PCs show heterogeneity depending on their position within the vasculature. As a result, PC interactions with surrounding endothelial cells, astrocytes, and neuron cells play a key role in a wide array of neurovascular functions such as regulating blood–brain barrier (BBB) permeability, cerebral blood flow, and helping to facilitate the clearance of toxic cellular molecules. Therefore, a reliable method of engineering brain-specific PCs from human induced pluripotent stem cells (hiPSCs) is critical in neurodegenerative disease modeling. This review summarizes brain-specific PC differentiation of hiPSCs through mesoderm and neural crest induction. Key signaling pathways (platelet-derived growth factor-B [PDGF-B], transforming growth factor [TGF]-β, and Notch signaling) regulating PC function, PC interactions with adjacent cells, and PC differentiation from hiPSCs are also discussed. Specifically, PDGF-BB-platelet-derived growth factor receptor β signaling promotes PC cell survival, TGF-β signal transduction facilitates PC attachment to endothelial cells, and Notch signaling is critical in vascular development and arterial-venous specification. Furthermore, current challenges facing the use of hiPSC-derived PCs are discussed, and their ongoing uses in neurodegenerative disease modeling are identified. Further investigations into PCs and surrounding cell interactions are needed to characterize the roles of brain PCs in various neurodegenerative disorders.
Impact statement
This article summarizes the work related to brain-specific pericytes (PCs) derived from human pluripotent stem cells (hPSCs). In particular, key signaling pathways regulating PC function, PC interactions with adjacent cells, and PC differentiation from hPSCs were discussed. Furthermore, current challenges facing the use of hPSC-derived PCs were identified, and their ongoing uses in neurodegenerative disease modeling were discussed. The review highlights the important role of cell–cell interactions in blood–brain barrier (BBB) models and neurodegeneration. The summarized findings are significant for establishing pluripotent stem cell-based BBB models toward the applications in drug screening and disease modeling.
Keywords: human pluripotent stem cells, pericytes, mesenchymal stem cells, brain specific, neuroinflammation
Background: What Are Pericytes?
Pericytes (PCs) are a type of perivascular cells that surround endothelial cells of small blood vessels and embedded in the basement membrane on the capillary wall.1 The brain has the highest ratio of PCs to endothelial cells, and PC deficiencies can cause severe neurological pathologies.2 The density of PCs along the vasculature in the brain is about one PC per 3–5 endothelial cells, while the ratio is one PC per 10–100 endothelial cells in skeletal muscle.2 PCs have elongated stellate-shaped processes that ensheath the capillary wall, exhibiting more circumferential processes at the arteriole end, more longitudinal processes in the middle of capillary, and a stellate morphology at the venule end of capillary.3 PCs play an essential role in remodeling and stabilization of developing blood vessels by forming tight junctions with endothelial cells.4,5 In particular, brain PCs are considered as tissue resident mesenchymal stem cells (MSCs) as they exhibit multipotent properties similar to MSCs and are able to differentiate into osteoblasts and adipocytes.6
Since different subclasses of PCs contribute differently to cerebral malfunction in various diseases, PCs are classified into three categories3: (i) PCs that are closer to the arteriole end and involved in regulating cerebral blood flow. Usually these cells express more α-smooth muscle actin (αSMA). (ii) PCs in the middle of the capillary bed, expressing less αSMA and important for maintaining the integrity of blood–brain barrier (BBB) that is damaged after brain injury (e.g., stroke) and neurodegeneration (e.g., Alzheimer's disease); and (iii) PCs at the venule end, playing a critical role in regulating immune cell entry to the brain parenchyma.1
There have been significant debates about what defines PCs due to their plasticity and the overlapping phenotype with MSCs.7–9 PCs do not have a unique expression profile and lack of definitive markers. Therefore, it is difficult to distinguish PCs from closely related cell types, that is, vascular smooth muscle cells (vSMCs) and MSCs. Common markers used to identify PCs include platelet-derived growth factor receptor β (PDGFRβ), neural/glial antigen 2 (NG2), CD146, CD105, CD13, desmin, collagen IV, etc.10 Similarly, PCs are also known to contain cell-to-cell junction proteins, for example, N-cadherin, the adherens junction protein, and connexin 43, and express several adhesion molecules, including vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1). The roles of PCs in the neurovascular unit have been illustrated elsewhere.11 PCs are involved in 13 signaling pathways, which interact with each other. The key signaling pathways (e.g., Notch, transforming growth factor [TGF] β signaling, and vascular endothelial growth factor [VEGF] signaling) are converged due to PC-endothelial cell and PC-astrocyte interactions. These pathways contribute to PC dysfunction in various neurological disorders.12
The Role of Brain-Specific Pericytes
In the central nervous system (CNS), PCs serve as the critical coordinators, integrators, and effectors of neurovascular function.13,14 They regulate cerebral blood flow, BBB permeability, and clearance of toxic cellular molecules. There are two origins of brain-specific PCs: (i) mesoderm-derived MSCs and (ii) neuroectoderm-derived neural crest cells.13 It was found that the neuroectoderm gave rise to PCs in the forebrain and the mesoderm progenitors gave rise to PCs in the midbrain, hindbrain/spinal cord, and peripheral organs.15–17 The heterogeneity of brain PCs is indicated by the regional differences in the CNS,18,19 and high PC coverage was observed for cortex and hippocampus but low PC coverage was shown for spinal cord.
Despite that, PCs display region-specific heterogeneity in the human brain depending on their positions; it has also been suggested that certain subpopulations can share location and origin while displaying differences in phenotype and functional response. Park et al. isolated two PC subpopulations originating in the same location from human brains by differential CD90 expression.20 The CD90+ population appeared primarily responsible for remodeling vasculature and replenishing lost cells during angiogenesis, while the CD90− population maintained neurovasculature and regulated immune response and vessel diameter.
The role of brain PCs in the blood-brain barrier
The BBB, as a highly selective semipermeable barrier, separates the circulating blood from the brain and extracellular fluid.21 The BBB is composed of endothelial cells, PCs embedded in the capillary basement membrane, and astrocytes with the end-feet enclosing the capillary. The BBB works to restrict the passage of pathogens and the large (or hydrophobic) molecules into the interstitial fluid, as well as the solute diffusion from the blood,22–24 but still allowing the diffusion of small molecules (such as oxygen, carbon dioxide, and hormones). PCs are a critical component of the BBB in the human brain and are mainly responsible for maintaining its integrity.21 In the BBB, the absence of PCs increases permeability since PCs secrete various mediators that affect transporter and tight junction proteins in brain microvascular endothelial cells (BMECs).25 PCs also regulate the extracellular matrix (ECM) composition in the basement membrane and help to direct the attachment of astrocyte end-feet to the basement membrane.
Loss of brain PCs reduces tight junction protein expression, for example, Occludin, claudin 5, and zonula occludens-1 (ZO-1), and increases transendothelial fluid flow and paracellular transport.26–28 The vascular dysfunction then leads to neural degeneration.29,30 The heterogeneity of brain PCs also contributes to the heterogeneity of BBB structure.18,19 For example, BBB of arterioles exhibits lower P-gp levels, while the BBB of venules shows high and uniform P-gp levels. Consequently, the permeability of BBB in different brain regions demonstrates functional differences.18,19 However, further roles of PCs are yet to be determined since it is difficult to isolate brain PCs with their nonunique expression profiles.
In vitro BBB models have been reported using primary PCs along with other human induced pluripotent stem cell (hiPSC)-derived cells (in particular BMECs).31–36 In one study, human fetal brain astrocytes and PCs were cocultured with hiPSC-BMECs and neural progenitor cells to model the BBB.37 Upregulated BBB genes ABCB1, SLC1A1, SLC2A1 (i.e., GLUT-1), and OCLN (the gene encoding Occludin) were observed. Paracellular transport of small molecules through this in vitro BBB model was demonstrated, and transcellular passage of lipid-soluble agents was recapitulated. Six cell types, including hiPSC-derived cells (microglia, oligodendrocytes, and neurons) and human primary cells (BMECs, astrocytes, and PCs), were used to form three-dimensional (3D) spheroids for studying cellular response of inorganic mercury toxicity and the neurotoxicity to the small molecule that can induce Parkinson's disease.38 Instead of PCs, several studies have used hiPSC-derived BMECs with ECMs (i.e., collagen I gels, fibronectin, collagen IV, and laminin) to mimic basement membrane in BBB.39,40 It has been suggested that BMECs in monolayer do not need coculture with PCs to reach physiological transendothelial electrical resistance (TEER) values. However, coculture with PCs restored optimal TEER under stressed conditions.41
In our study, we found that coculturing MSCs with endothelial spheroids and cortical spheroids promoted the expression of BBB-related genes such as GLUT-1 (gene encoding glucose transporter 1) and BCRP (gene encoding breast cancer resistance protein).42 Similarly, a protocol was described for coculture of primary endothelial cells, PCs, and astrocytes as 3D organoids that reproduce some of the BBB properties, including the expression of tight junctions, drug efflux pumps, and molecular transporters.43 Furthermore, drug transport across the BBB was modeled using two detection approaches, mass spectrometry imaging, and confocal fluorescence microscopy, to analyze drug penetration into the organoids. Inclusion of PCs into brain organoids could prove as an invaluable tool for developing drugs that are able to penetrate the BBB and reach the CNS.
Markers for brain-specific PCs
Comparing with other mural cells, capillary-associated PCs generally express PDGFRβ, NG2, CD13, and CD146. In addition, high expression of PDGFRβ or αSMA is commonly considered as the indicator of PCs and vSMCs, respectively. NG2 and CD13 are not PC specific since they are also present on vSMCs in large vessels, and when colabeling with αSMA, CD146 and desmin can be detected in vSMCs too.44 Some new PC markers have been suggested but need to be validated, including RGS5 (gene encoding Regulator of G-protein signaling 5),45 ABCCP (SUR2), KCNJ8 (also known as Kir6.1, gene encoding potassium inwardly rectifying channel subfamily J member 8), Endosialin, and DLK1 (gene encoding Delta like noncanonical Notch ligand 1).5,46
To figure out how PCs in the CNS differ from those of other tissues (i.e., brain PCs vs. nonbrain PCs), the forkhead transcription factor FOXF2 was identified to be specifically expressed in PCs of the brain.47 FOXF2 inactivation in brain PCs resulted in faster proliferation and the reduced PDGFRβ expression. In addition, it was found that TGF-β-Smad2/3 signaling was attenuated, and the expression of TGF-β pathway components was reduced, such as TGF-β2, type 2 TGF-β receptor (TGFβR2), Alk5, and integrin αVβ8. By contrast, Smad1/5 and p38 phosphorylation was enhanced. BBB breakdown, increased transendothelial vesicular transport, and endothelial thickening were observed when FOXF2 was inactivated. Some other markers are also identified, including ZIC1 (gene encoding Zic family member 1), ABCC9 (gene encoding ATP-binding cassette subfamily C member 9), and KCNJ8.46,48 All these markers can be used as a panel to differentiate brain PCs from other mural cells.
Signaling pathways during PC-endothelial cell crosstalk
The major pathways during PC and endothelial cell crosstalk include the (i) platelet-derived growth factor-B (PDGF-B) pathway, (ii) TGF-β pathway, and (iii) Notch signaling pathway.13 These key signaling pathways are also involved in astrocytes and neurons, controlling neurovascular functions. Other notable pathways include the VEGF-A-VEGFR2 pathway, Angiopoietin-Tie2 pathway, and MFSD2A (a membrane transport protein expressed in the BBB) signaling pathway.11
The endothelial cells secrete PDGF-B as a homodimer PDGF-BB that has positively charged C-terminal electrostatically interacting with negatively charged heparin sulfate proteoglycans and resulting in localized PDGF-BB retention.13 The appropriate PDGF-BB concentration gradient is critical for PC recruitment, attachment, and migration.49 Binding of PDGF-BB to PDGFRβ results in the dimerization of receptors and the activation of downstream signaling pathways, for example, extracellular-signal-regulated kinase (ERK) and phosphatidylinositol 3-kinase (PI3K)-Akt pathways (Fig. 1). PDGF-BB-PDGFRβ signaling was found to promote PC survival, and PDGFRβ receptor abundance correlates with the number of PCs in the neural tube.13,50
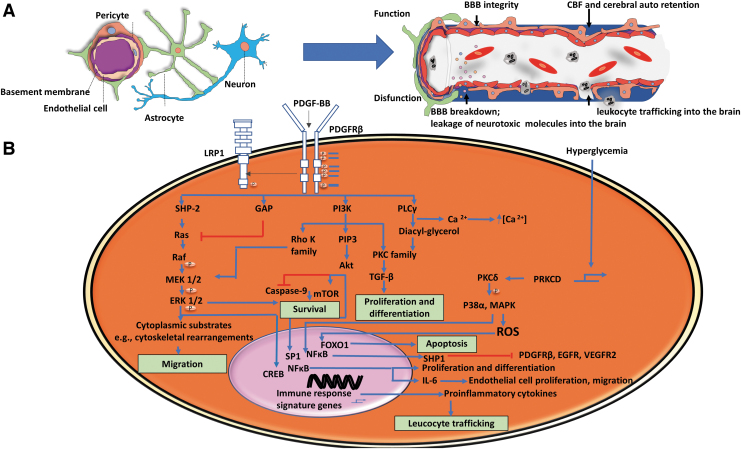
FIG. 1.
The role of brain pericyte function. (A) Overall schematic cell–cell interactions in BBBs: pericytes, endothelial cells, astrocytes, and neurons, and the role of pericytes in BBB. Functional pericytes maintain BBB integrity, while the pericyte loss leads to BBB breakdown. (B) The role of PDGF-BB-PDGFRβ pathway in pericyte function. Endothelial cells secrete PDGF-BB that binds to PDGFRβ, resulting in receptor dimerization and phosphorylation of tyrosine on the cytoplasmic side of the receptor. For example, Src homology 2 (SH2) domain-containing proteins (e.g., SHP-2, PI3K, etc.) bind to distinct tyrosine residues, activating signaling pathways and transcription factors that regulate cell survival (e.g., mTOR), differentiation (e.g., TGF-β), migration (e.g., ERK), proliferation (e.g., TGF-β), apoptosis (e.g., ROS and caspase), and leukocyte trafficking (induced by pro-inflammatory cytokines). Adapted from Sweeney et al.11 CREB, cAMP response element-binding protein; GAP, GTPase-activating proteins; PLC, phospholipase C; PKC, protein kinase C; PRKCD, protein kinase C delta signaling axis; BBB, blood–brain barrier; CBF, cerebral blood flow; ROS, reactive oxygen species; PI3K, phosphatidylinositol 3-kinase; ERK, extracellular-signal-regulated kinase; TGF, transforming growth factor; PDGF-B, platelet-derived growth factor-B; PDGFRβ, platelet-derived growth factor receptor β. Color images are available online.
Secreted by endothelial cells, PCs, glia, and neurons, TGF-β can induce PC differentiation and adhesion.51 The activated TGF-β binds to the TGFβR2, leading to the phosphorylation of activin-like kinase 1 or 5 (i.e., ALK1 or ALK5), type 1 TGF-β receptor, and the translocation of Smad2–3–4 protein complex (Fig. 2).13 TGF-β signal transduction is involved in PC attachment to endothelial cells. Coordinated with Notch signaling, adhesion molecule N-cadherin is upregulated.13 It was found that Smad deletion resulted in PC detachment and the reduction of PC coverage of the capillary.52 During the CNS development, PCs secrete TGF-β to activate the TGFβR2-ALK5-Smad2/3 pathway and promote endothelial cell maturation and BBB formation.11
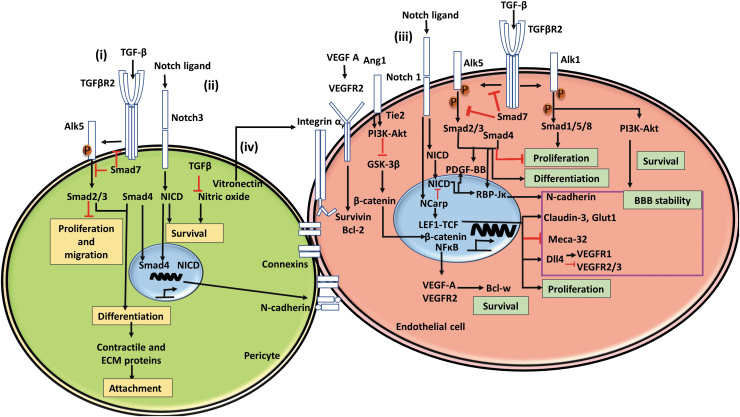
FIG. 2.
The interactions of pericytes with endothelial cells. (i) The roles of TGF-β pathway. In both pericytes and endothelial cells, TGF-β binds to TGFβR2, which subsequently phosphorylates Alk5, activating Smad signaling cascades that play a vital role in cell function such as proliferation, migration, differentiation, and expression of contractile and ECM proteins. In addition, TGF-β inhibits nitric oxides and promotes cell survival. (ii) The role of Notch signaling in pericytes and endothelial cells. Notch ligands bind notch, leading to translocation of NICD and the Smad cascade, which work synergistically to promote N-cadherin expression, increasing BBB stability. (iii) In endothelial cells, VEGF A binds VEGFR, affecting cell survival, proliferation, and differentiation. Furthermore, Alk5 works synergistically with Notch signaling and Tie2 downstream signals to promote BBB stability. Pericytes directly affect this process through Ang1-Tie2 signaling. (iv) Pericytes also secrete vitronectin, which interacts with integrin αv, resulting in transcriptional activities. Adapted from Sweeney et al.11 ECM, extracellular matrix; VEGF, vascular endothelial growth factor; NICD, Notch intracellular domain. Color images are available online.
Notch signaling plays an important role in vascular development and arterial-venous specification.11 There are four Notch receptors, Notch 1–4, and five Notch ligands, that is, Jagged 1/2 and delta-like 1, 3, and 4. The ligand-receptor binding leads to the proteolytic cleavage that releases Notch intracellular domain (NICD).53 NICD translocation into the nucleus results in downstream transcriptional changes.54 Notch signaling interacts with other signaling pathways such as Wnt54 and is important for PC attachment to the endothelial cells and cell survival.13 However, the exact role of Notch signaling in PC function is yet to be elucidated.
PC interactions with other cell types
PC-endothelial cell interactions
PCs are proposed to play two roles in the BBB: regulating the expression patterns of BBB-specific genes in endothelial cells and inducing polarization of astrocyte end-feet that surrounds CNS blood vessels.55 PC coverage is directly correlated with BBB integrity, that is, more PCs giving rise to better BBB structure.56 Conversely, lower PC coverage was reported to reduce CD71 expression in brain-specific endothelial cells and lead to increased endothelial transcytosis.11,57 PCs promote the formation of tight junctions and vesicle trafficking in endothelial cells, regulating BBB function.58 In addition, differential release of cytokines granulocyte-colony stimulating factor (G-CSF), interleukin (IL)-6, and IL-8 in 3D BBB chips was observed in coculturing endothelial cells with astrocytes or PCs.59
PC-astrocyte interactions
Astrocytes secrete apolipoprotein E (APOE), interacting with cell-surface low-density lipoprotein receptor-related protein-1 (LRP1) on PCs.60 The APOE-LRP1 pathology regulates the activation of pro-inflammatory cyclophilin A and matrix metalloproteinase (MMP)-9 in hippocampal and cortical PCs and endothelial cells in an APOE4 (but not APOE3) dependent manner.61 The APOE4-induced BBB breakdown enables the cross of plasma proteins immunoglobulin G and fibrin and causes neurodegeneration. Increased intracellular Ca2+ transients in PCs through interactions with astrocytes62 were shown to promote cell contraction. An increase in extracellular K+ was found to activate Ca2+ channels and increase intracellular Ca2+ levels, leading to depolarization and contraction of primary rat brain PCs.11
Arachidonic acid can be metabolized in astrocytes to produce prostaglandin E2 (PGE2) by cyclooxygenase-1, which regulates the responses of PCs.11 PGE2 can activate PGE2 receptor 4 in PCs and induce PC relaxation after glutamate addition.63 Arachidonic acid secreted by astrocytes can also be metabolized in PCs by membrane-bound CYP4A.11 It was reported that PC degeneration diminishes capillary cerebral blood flow responses to neuronal stimuli, leading to reduced oxygen supply, metabolic stress, and neurovascular uncoupling.64
PC-neuron interactions
PCs can regulate neuronal function through secreted cytokines and factors. PCs (i.e., PDGFRβ+ cells) secrete chemokine C–C motif chemokine ligand 2, which can promote excitatory synaptic transmission in glutamatergic neurons of different brain regions upon lipopolysaccharide (LPS) stimulation.65 Cultured brain PCs were reported to secrete neurotrophic factors at the levels comparable to cultured astrocytes.59,66 The PC-conditioned media were found to increase the Akt phosphorylation simulated by insulin and insulin receptor β phosphorylation, suggesting that PCs can release soluble factors to increase the sensitivity of hypothalamic neurons to insulin.11,67 These results indicate that PCs function as initial sensors of external insults and then relay the signals to the CNS. In the hippocampus of mice, the average distance between a neuron and a capillary is about 8–23 μm, while the average distance between a neuron and an arteriole is 70–160 μm.68 This suggests that, comparing to vSMCs, PCs respond earlier to changes in neuronal activity and receive chemical transmitters faster from activated neurons.69 Conversely, neuron-secreted factor norepinephrine may cause PC contraction and the reduction of the diameter of capillaries.70 Neurotransmitters, including gamma-aminobutyric acid, glutamate, and dopamine, can lead to PC relaxation.11
Metabolic regulation of PC phenotype
There are many similarities in metabolism shifts with PC phenotype compared to MSCs and microglia.71,72 For these cells, glycolysis is associated with more primitive phenotype, while the lineage-committed differentiation uses more oxidative phosphorylation, showing the upregulation of mitochondrial capacity.73–77 In particular, Notch signaling is involved in the regulation of PC metabolism and quiescence, for example, through the downregulation of glycolytic enzymes (e.g., PFKFB3).78 PDGF-BB is a strong driver of glycolysis, and therefore, PDGFRβ signaling may enhance glycolysis in proliferating PCs to meet the high demands of energy and biomass production.79 Excess nutrients affect PC metabolism. For example, increased levels of glucose enhance glycolysis and increased flux in polyol pathway consumes nicotinamide adenine dinucleotide phosphate (NADPH). Hyperglycemia elevates flux through the hexosamine pathway, and increased levels of fatty acids result in insulin resistance and lipid toxicity.71
The Roles of Brain PCs in Neuroinflammation
Comparing to endothelial cells, PCs express similar patterns of transcription factor activation in response to inflammatory stimuli (IL-1β, tumor necrosis factor [TNF]-α, LPS, interferon [IFN]-γ, TGF-β1, IL-6, and IL-4); however, the secretion levels are different.80 Several cell-type specific responses were identified, including the secretion of G-CSF, granulocyte-macrophage colony-stimulating factor (endothelial specific), as well as the expression of insulin-like growth factor-binding protein (IGFBP) 2 and IGFBP3 (PC specific). In particular, brain PCs act as a critical mediator at the brain–immune interface.4,81 During neuroinflammation, brain PCs participate in six categories of the cellular events,4,13,80 including the recruitment of leukocytes, BBB disruption, endocytosis, response to inflammatory stimuli, the polarization of microglia, and adaptive immunity (Fig. 3).
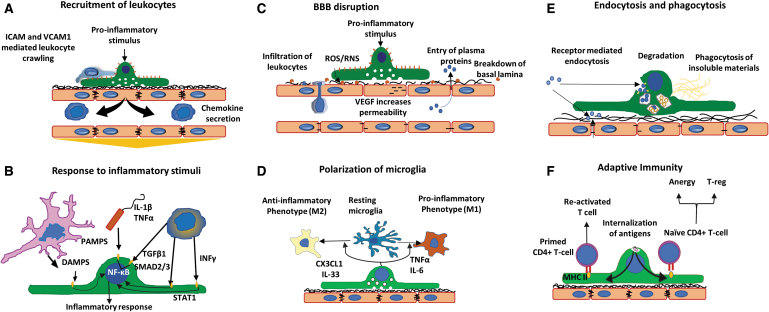
FIG. 3.
Brain pericytes as mediators of neuroinflammation. (A) Recruitment of leukocytes. Pericytes secrete chemokines to actively recruit leukocytes to areas of inflammation. Leukocytes cover gaps between pericytes and utilize ICAM-1 and VCAM-1 guidance to enter the brain parenchyma. (B) Response to inflammatory stimuli. Receptors for PAMPs, DAMPs, and cytokines facilitate pericyte response to pro-inflammatory stimuli. (C) BBB disruption. A variety of factors such as ROS, RNS, and VEGF can promote the breakdown of endothelial tight junctions and basement membrane, leading to BBB disruption. (D) Polarization of microglia. Secretion of pro- or anti-inflammatory mediators by pericytes can alter phenotype for nearby microglia (M1 or M2, respectively). (E) Endocytosis and phagocytosis. Receptors on the membrane detect insoluble materials and dead cells to facilitate their clearance. (F) Adaptive immunity. Internalized antigens from pericytes to primed or naive CD4+ T cells promote T-reg, T cell anergy, or T cell activation. Adapted from Rustenhoven et al.4 PAMP, pathogen-associated molecular pattern; DAMP, damage-associated molecular pattern; RNS, reactive nitrogen species; ICAM-1, intercellular adhesion molecule-1; VCAM-1, vascular cell adhesion molecule-1. Color images are available online.
For the recruitment of leukocytes, PCs (like microglia) can secrete chemokines that attract circulating leukocytes to the brain when stimulated by pro-inflammatory factors, such as TNF-α, IL-1β, LPS, IFN-γ, and TGF-β1.81 PCs also secrete CC chemokines, membrane cofactor protein-1, the CX3 chemokine ligand 1 (CXCL1), and CX3CL1,4 and express adhesion molecules ICAM-1 and VCAM-1, which enable leukocyte adhesion and transverse (Table 1).82 The pro-inflammatory factors stimulate the expression of ICAM-1 and VCAM-1, induce matrix remodeling of collagen IV and laminin-511,52 and elevate the levels of nitric oxide (NO) and PGE2.83
Table 1.
Summary of Molecules, Factors, and Mediators Expressed by Pericytes
| Factor categories | Immune mediators and properties |
|---|---|
| Adhesion molecules | ICAM-1, VCAM-1, MCAM, N-cadherins |
| Chemokines | CCL2 (MCP-1), CCL3, CCL4, CCL5, CCL11, CXCL1, CXCL8, IL-8, CXCL10 (IP-10), CX3CL1 |
| Cytokines | IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12, IL-13, IL-17, IL-18, IL-33, IFN-γ, TNF-α, G-CSF, GM-CSF |
| Antigen presentation | MHC-II, HLA-DR |
| ROS/RNS | iNOS/NO, NOX4/O2− |
| Transcription factors | NF-kB, C/EBPδ, STAT1, SMAD2/3 |
| Phagocytic/endocytic receptors | Fc receptor, CR3, CD36, CD47, CD68, LRP-1 |
| MMPs | MMP2, MMP9 |
| ECM adhesion | Prefer fibronectin over laminin; express fibronectin-binding integrins |
| Mechanical regulation | Provide mechanical strength to the endothelium |
| Sense substrate stiffness | Respond to suboptimal stiffness with increased traction forces, altered cytoskeleton, and decreased spreading. Optimal substrate stiffness: 30–50 kPa |
CR3, complement receptor 3; ECM, extracellular matrix; G-CSF, granulocyte-colony stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; IP-10, IFN-γ induced protein 10; LRP-1, low density lipoprotein receptor-related protein 1; MCAM, melanoma cell adhesion molecule; MMP, matrix metalloproteinase; ROS, reactive oxygen species; RNS, reactive nitrogen species; NO, nitric oxide; VCAM-1, vascular cell adhesion molecule-1; iNOS, inducible NO synthase; ICAM-1, intercellular adhesion molecule-1; IL, interleukin; TNF, tumor necrosis factor; IFN, interferon.
PCs can respond to inflammatory stimulation and propagate CNS inflammation. PCs express receptors for pathogen-associated molecular pattern molecules, such as Toll-like receptors, which recognize bacteria and certain viruses to induce inflammatory response.84 A member of CCAAT-enhancer-binding protein (C/EBP) family, C/EBPδ, reduces expression of adhesion molecules and chemokine secretion in PCs.85 Induction of the inflammatory mediators on PCs involves the nuclear factor-kB pathway (induced by LPS, IL-1β, and TNF-α) and STAT1-Smad2/3 pathway (induced by IFN-γ and TGF-β1).80
For the role in BBB disruption, a lack of PCs was found to enhance BBB permeability and allow the entrance of blood-derived products such as fibrinogen.86 Fibrinogen-fibrin infiltration leads to the activation of microglia and T cells, resulting in axonal degeneration.87 When the BBB breaks down due to PC loss, the remaining PCs can produce reactive oxygen species, for example, NO and NOX-4-derived superoxide secrete pro-inflammatory cytokines and promote apoptosis. In addition, increased MMP2 and MMP9 activate VEGF signaling and enhance BBB permeability through ZO-1 and occludin rearrangement.88,89 Brain PCs treated with pro-inflammatory cytokines, for example, IL-1β and TNF-α, decrease the expression of fibronectin, PDGFRβ, and connexin 43.90 Hypoxia and Aβ40/42 were also found to promote membrane shedding of NG2 and PDGFRβ from PCs that leads to PC dysfunction.91
PCs can secrete pro-inflammatory factors TNF-α, IFN-γ, IL-1β, and IL-6 to affect the polarization state of adjacent endothelial cells, microglia (i.e., referred as M1 polarization), and astrocytes (i.e., referred as A1 polarization).92 The polarization state of the cells was recently found related to the metabolic state of the cells.93 The cells also can secrete anti-inflammatory factors such as CX3CL1 and IL-33 through PDGF-BB stimulation, that is, M2 or A2 polarization. For example, IL-33 and CX3CL1 can prevent microglia activation in Alzheimer's disease and promote neuroprotective microglia phenotype.94 The brain PCs were found to serve as microglia-generating multipotent vascular stem cells under pathological conditions of ischemic stroke.95
Through endocytosis and phagocytosis, PCs can clear soluble Aβ mediated by LRP1,96 as well as the aggregated form of Aβ40/42 to prevent plaque formation.97 Therefore, PC loss directly contributes to the accumulation of vascular Aβ deposits. Following ischemic stroke, the phagocytosis ability of brain PCs is hindered, and the cells exhibit decreased expression of PC markers CD13, αSMA, and PDGFRβ.98 However, brain PCs can adapt to tissue injury with the increased expression of microglial markers, IBA1, MHC-II, CD11b, and CD68, as well as the enhanced phagocytosis ability due to “microglia-like: phenotype.”98 This “microglia-like” phenotype may be beneficial to the clearance of compromised cells.
For adaptive immunity, PCs display pattern recognition receptors and can present antigens to CD4+ T cells through MHC-II-T cell receptor binding.99 Pattern recognition receptors facilitate the uptake of antigens and IFN-γ induced MHC-II and HLA-DR complexes.100 PCs can promote immunosuppressive T-reg populations, activating an anti-inflammatory phenotype,4 and stimulate the cytokine secretion by Th1 lymphocytes.92 However, this function may require the inductive cytokines (e.g., IL-12) and costimulatory molecules (e.g., CD86 and CD80).
PC Differentiation from Human Induced Pluripotent Stem Cells
The initial differentiation protocols for PC differentiation from human pluripotent stem cells (hPSCs), including human embryonic stem cells (hESCs) and hiPSCs, are based on spontaneous embryoid body formation.101–104 While this protocol generated the cells expressing some PC markers (CD146, CD105, and PDGFRβ), the yield was very low. Due to the two origins of PCs, mesoderm and neural crest,105 two types of PC-specific differentiation protocols from hPSCs have been reported (Table 2).
Table 2.
Summary of Differentiation Protocols for Human Pluripotent Stem Cell-Derived Pericytes
| Cell source | Differentiation methods | Characterizations | References |
|---|---|---|---|
| ESI-017 hESCs | Spontaneous EB differentiation; EC medium | CD146, CD105, PDGFRβ | Greenwood-Goodwin et al.102 |
| H9, I6 hESCs | Spontaneous EB differentiation | CD105, NG2, PDGFRβ, calponin, nestin | Dar et al.103 |
| C3, KTR13 hiPSCs | |||
| NL-HES4 hESCs | Mesoderm induction: BMP4, Activin A, VEGF | CD105, NG2, PDGFRβ, CD146, CD73, CD44, 30% yield; functional in cultured vascular plexus and zebrafish xenografts | Orlova et al.; Iendaltseva et al.106,120,139 |
| hiPSCs | |||
| H9 hESCs | Mesoderm induction: 10% serum in α-MEM; then VEGF and SB431542 in EC medium | NG2, PDGFRβ, CD73, CD44; studied the differences compared to vSMCs (SMA, SMMHC, calponin). ECM protein production, collagens and elastin, MMP2, 9, 14, migration and invasion | Wanjare et al.108 with Jamieson et al.41 |
| hiPSCs | |||
| H1 hESCs | Mesoderm induction: BMP4, VEGF, FGF-2 in XVIVO-15 medium; later using SB431542; CD325-CD56+ cells were sorted using FACS | The derived cells coexpressed common mesenchymal markers (CD73, CD105, CD90, and PDGFRβ) with a subset of cells defined as CD146hiCD73hi. Transcriptional similarity exists between hPSC-derived CD146++ and primary human CD146++ perivascular cells | Chin et al.111 |
| UCLA3 and UCLA6 hESCs | |||
| H1, H9 hESCs | Mesoderm induction: coculture with OP9 stromal layer; or with BMP4, Activin A, and FGF-2; with BMP4, CHIR99021, PDGF-BB, and Activin A | MBs differentiate into primitive PDGFRβ+CD271+CD73− mesenchymal progenitors, which give rise to proliferative PCs, SMCs, and MSCs. MB-derived PCs can be further specified to CD274+ capillary and DLK1+ arteriolar PCs | Kumar et al.110 Blanchard et al.138 |
| hiPSCs | |||
| H9 hESCs | Mesoderm induction: MIM-mPCs | NG2, PDGFRβ, CD13, CD146; brain specific markers VTN, FOXC1, FOXF2. FOXF2 is higher in ncPCs than mPCs Functional assay: coculture with ECs forms tube-like structures; Wnt inhibition by DKK1 reduced PAX7 expression |
Faal et al.115 |
| hiPSCs from AD patients, lines AD 5, 6 (APOE4), 13, 14, 20, 22 (APOE3), 29 | Neural crest induction: Wnt activation using CHIR99021, DMEM/F12 plus B27-ncPCs | ||
| H9 hESCs | Neural crest induction: Wnt activation using CHIR99021, plus SB431542, FGF2, dorsomorphin, heparin in E6 medium; use MACS sorting and 10% FBS | PDGFRβ, NG2, expressing CNN1, TAGLN, ANPEP, TBX18; brain mural markers: FOXF2, ZIC1, ABCC9, KCNJ8; functional assay: the derived cells assemble with vascular cord networks; induced BBB properties | Stebbins et al.118 |
| IMR90C4 and CS03n2 iPSCs |
DKK1, Dickkopf WNT signaling pathway inhibitor 1; FBS, fetal bovine serum; MACS, magnetic-activated cell sorting; SMMHC, smooth muscle myosin heavy chain; PC, pericyte; BBB, blood–brain barrier; hiPSC, human induced pluripotent stem cell; hESC, human embryonic stem cell; FGF, fibroblast growth factor; MIM, mesodermal induction medium; BMP4, bone morphogenetic protein 4; PDGF-B, platelet-derived growth factor-B; FACS, fluorescence-activated cell sorting; VEGF, vascular endothelial growth factor; EC, endothelial cell; EB, embryoid body; PDGFRβ, platelet-derived growth factor receptor β; NG2, neural/glial antigen 2; vSMC, vascular smooth muscle cell; SMA, smooth muscle actin; hPSC, human pluripotent stem cell; MB, mesenchymoangioblast; MSC, mesenchymal stem cell; α-MEM, alpha-minimal essential medium; DMEM, Dulbecco's modified Eagle's medium.
Mesoderm induction
Mesoderm induction was performed using bone morphogenetic protein 4 (BMP4), Activin A, and VEGF through activation of BMP, Activin, and VEGF signaling. This method generated the cells expressing CD105, NG2, PDGFRβ, CD146, CD73, and CD44 with a 30% yield.41,106,107 Similarly, α-minimal essential medium plus 10% serum followed by VEGF and SB431542 (the TGF-β superfamily type I activin receptor-like kinase inhibitor) in endothelial cell culture medium produced cells expressing NG2, PDGFRβ, CD73, and CD44, which were different from vSMCs.108 The derived cells were capable of secreting ECM proteins, such as collagens and elastin, as well as ECM remodeling proteins MMP2, 9, 14. The migration ability and invasion capability of the derived cells were also confirmed. Analysis of hPSC differentiation into mesoderm has suggested that cell cycle regulators play an essential role in early stage development, and the differentiation is regulated through corresponding developmental pathways such as the fibroblast growth factor (FGF)/ERK pathway.109
A similar method reported that hPSCs were first differentiated into the stage of mesenchymoangioblast (MB) using BMP4, Activin A, and FGF-2.110 The derived mesenchymal progenitors had the PDGFRβ+ CD271+ DLK1+ CD73− phenotype. Since PDGF-B/PDGFRβ signaling plays a vital role in PC development in vivo, treatment with PDGF-B would elicit the PC differentiation potential. This cell population can give rise to proliferative PCs, SMCs, and MSCs. MB-derived PCs can be specified to CD274+ capillary type (showing a pro-inflammatory phenotype) and DLK1+ arteriolar type (showing contractile phenotype).110 In addition, the hPSCs were induced for mesoderm differentiation using OP9 stromal cell coculture or growth factors BMP4, VEGF, and FGF-2.111 The derived cells coexpressed common mesenchymal markers (CD73, CD90, CD105, and PDGFRβ), and a subset of CD146hiCD73hi population was identified. It was found that the derived cells provided stromal support of hematopoietic stem cells, which was contact dependent and mediated through high Notch signaling (e.g., Jagged 1) and low Wnt signaling. Transcriptional similarity was demonstrated between hPSC-derived CD146hi cells and primary human CD146hi perivascular cells through molecular profiling.
An alternative approach for mesoderm induction is to generate the multipotent vascular progenitor (MesoT) cells from hPSCs, which can give rise to SMCs, endothelial cells, and PCs, which can self-assemble into vessel-like networks in vitro.112,113 The differentiation is induced using Wnt3a and BMP4 along with retinoic acid for further differentiation into MesoT cells. The differentiation of MesoT cells to SMCs or endothelial cells was promoted by PDGF-BB or with a combination of VEGF and SB431542, respectively.
Neural crest induction
Neural crest is a transient multipotent cell population and emerges between the developing stages of neural plate and non-neural surface ectoderm,114 which is capable of forming peripheral neurons. One study showing PC differentiation from hiPSCs through neural crest induction used canonical Wnt signaling activation by CHIR99021, a chemical compound which acts as an inhibitor of the enzyme Glycogen synthase kinase-3, in Dulbecco's modified Eagle's medium:Nutrient Mixture F-12 plus B27 medium.115 The expression of NG2, PDGFRβ, CD13, CD146 and brain-specific markers VTN (gene encoding vitronectin), FOXC1 (gene encoding Forkhead box C1), FOXF2 was observed. FOXF2 expression is higher in neural crest-induced PCs than mesoderm-induced PCs. Similar to mesoderm induction, the derived cells were capable of increasing endothelial barrier properties and vascular tube formation. This protocol is similar to endothelial cell differentiation of hiPSCs through Wnt activation,116 although the concentration and the duration of Wnt activator CHIR99021 treatment are very different, resulting in generation of the cells originated from two different germ layers.
Multipotent neural crest cells, expressing PAX7 (gene encoding Paired Box 7), SOX10 (gene encoding SRY-Box Transcription Factor 10), and TFAP2A (gene encoding transcription factor AP-2 alpha), were generated from hESCs through activation of Wnt/β-catenin signaling.117 The canonical Wnt/β-catenin signaling is responsible for distinct responses to FGF and BMP signaling from a preneural border intermediate. By contrast, Activin/TGF-β antagonism (e.g., SB431542) does not promote human neural crest induction.
To better mimic the BBB in vitro using hPSCs, forebrain PCs were derived from hPSCs in E6 medium supplemented with CHIR99021 (for Wnt activation), SB431542 (Activin/TGF-β1 inhibitor), FGF-2, dorsomorphin (a BMP type I receptor inhibitor), and heparin (for growth factor stabilization) through neural crest induction.118 After magnetic-activated cell sorting (to purify neural crest progenitors) and using 10% fetal bovine serum, the progenitor cells were directed into mural lineages. The derived cells expressed PDGFRβ and NG2, as well as genes of CNN1, TAGLN, ANPEP, and TBX18. They also expressed brain mural markers FOXF2, ZIC1, ABCC9, and KCNJ8, showing a transcriptome similar to primary human brain PCs.
Current challenges for hiPSC-derived PCs
Currently, hiPSC-derived PCs have not been well characterized. Investigations up to this point have mainly focused on the expression of phenotypic markers (e.g., NG2, PDGFRβ, and CD146) and transcriptome comparison. Maturation of the derived cells (more or less at fetal stage of the development) to the primary adult brain PCs has not been demonstrated. Immunomodulatory abilities of hiPSC-derived brain PCs, as well as the roles of major signaling pathways (e.g., PDGF-BB, TGF-β, and Notch) in the derived cells, are yet to be investigated. In particular, their abilities as mediators of neuroinflammation remain unknown. Therefore, the applications of hiPSC-derived PCs are mainly in disease modeling12 and establishing the micro-physiological systems for drug screening or toxicity study,119 not in transplantations. Similarly, the secretome of hiPSC-derived PCs under homeostatic or stimulated conditions has not been well characterized. Some functional analysis has been performed, including vascular tube formation, migration ability, and invasion capability; however, results are preliminary.
A recent investigation starts to understand the physiology of hiPSC-derived pericytes.120 The results indicate that PCs use fibronectin patches (rather than laminin) as the anchoring points to sense force and conduct force transmission. The cells responded to suboptimal stiffness by showing the increased traction forces, decreased spreading, and altered cytoskeleton distributions. The optimal substrate stiffness for hiPSC-derived pericytes is 30–50 kPa. This study indicates that the mechanoresponsive behaviors of pericytes significantly differ from fibroblasts and other cell types.
Our studies showed the presence of brain PC phenotype in hiPSC-derived dorsal cortical organoids based on transcriptome analysis.72,121 Astrocytes and endothelial cells were also derived from hiPSCs for potential coculture experiments with isogenic brain PCs for neural degenerative disease modeling.42,122 Assembling vascular spheroids with isogenic brain organoids may enhance the role of PCs.42 Our study derived brain PCs from hiPSCs that expressed PDGFRβ and NG2 (Fig. 4). In addition, the brain identity indicated by ZIC1, KCNJ8, FOXF2, and ABCC9 gene expression demonstrates that the cortical organoids contain the cells with brain PC phenotype.
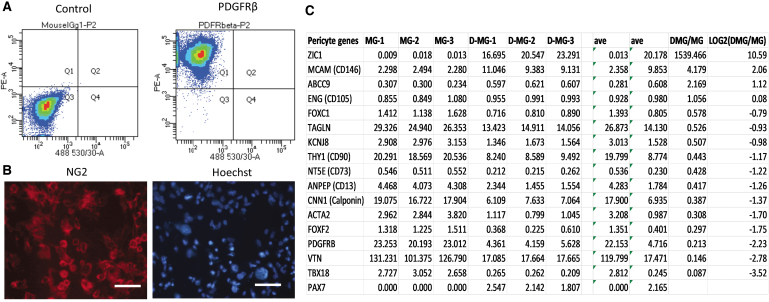
FIG. 4.
Brain pericytes derived from human pluripotent stem cells. (A) Flow cytometry result showing the positive expression of PDGFRβ in the derived cells. (B) Immunofluorescence images of NG2 expression. Scale bar: 50 μm. (C) A list of expressed genes associated with brain pericytes in cortical organoids with isogenic microglia.121 The FPKM normalized values for these genes are listed for both samples (MG, microglia-like cell; D-MG, cortical organoids with microglia). The numbers are the Log2 values of ratios of D-MG to MG. Negative values indicate that the genes are present in higher amounts in MG group, while positive values indicate that the genes are present in higher amounts in D-MG group. NG2, neural/glial antigen 2; FPKM, fragments per kilobase per million reads. Color images are available online.
Neural Degenerative Disease Modeling Based on hiPSC-Derived PCs
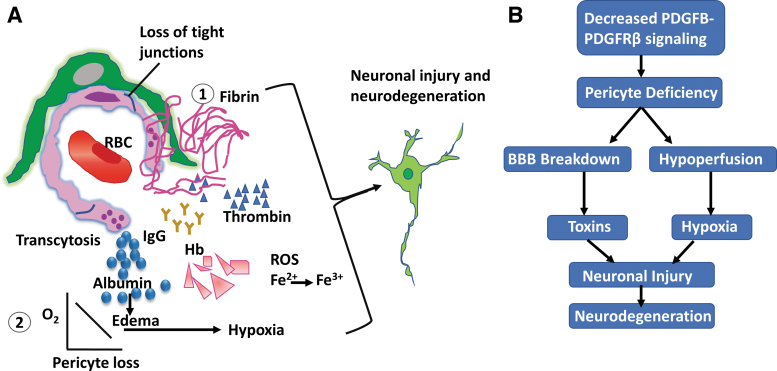
Neurovascular dysfunction can lead to neural degeneration.22,59,123,124 The loss of brain PCs can trigger two parallel pathways:28,125 (i) BBB breakdown, allowing the entrance of circulating plasma proteins, and (ii) chronic hyperfusion and hypoxia (Fig. 5), which can cause the secondary neurodegenerative phenotype. Brain PCs can protect the CNS from injury by maintaining cerebral vascular homeostasis. In Alzheimer's disease, it is known that amyloid β peptides accumulate around the capillaries causing PC cell death.126,127 Yet it is unclear how Aβ influences BBB protection and PC-mediated capillary contractility. It is suspected that transcriptional changes in PCs during Alzheimer's disease progression result in cerebral artery hypercontraction and the reduction of Aβ clearance.56
FIG. 5.
The role of brain pericytes in neurodegeneration. (A) ① Decreased PDGFB-PDGFRβ signaling leads to pericyte deficiency, a loss in homeostasis, and disruption of tight junctions and basement membrane proteins. This BBB degradation results in increased fluid flow and loss of selectivity of proteins and macromolecules. Neurotoxins such as thrombin, fibrin, and Hb cross BBB and cause neurodegeneration. The cross of BBB for serum proteins such as albumin and IgG results in edema. ② The microvascular degradation and pericapillary edema reduce capillary blood flow and lead to hypoxia. (B) A schematic depicting that reduction in PDGFB-PDGFRβ signaling leads to pericyte loss. The pericyte deficiency results in BBB breakdown and hypoperfusion, which contribute to neuronal injury and neurodegeneration. Adapted from Winkler et al.13 Hb, hemoglobin; IgG, immunoglobulin G; RBC, red blood cell. Color images are available online.
PC transplantation into the brains of Aβ precursor protein/Presenilin-1 (PSEN1) mice, an amyloid model for Alzheimer's disease, showed the increased brain microcirculation.128 The significantly lower levels of insoluble Aβ40 and Aβ42 were also observed in the hippocampus of the transplanted mice compared to the vehicle control. While APOE lipoprotein receptor LRP1 mediates Aβ cellular uptake, LRP1 knockdown did not change the amounts of major Aβ-degrading enzymes. PSEN1 and PSEN2 mutations are the leading cause of autosomal-dominant Alzheimer's disease.129,130 The mutations reduce the level of PDGFRβ expression, the binding sites of PDGF-BB, and the activation and autophosphorylation of PDGFRβ.13 As a result, the downstream pathways were suppressed, including the PI3K–Akt signaling and the mitogen-activated protein kinase–ERK pathway.
Most current hiPSC-based BBB models of multiple cell types coculture hiPSC-derived BMECs and primary PCs or culture hiPSC-BMECs in the absence of PCs.37,131,132 A polydimethylsiloxane microfluidic system in fibrin gel was used to culture hiPSC-ECs, PCs, and astrocytes, exhibiting perfusable and selective microvasculature. Compared to conventional in vitro models, the permeability of this BBB model was much lower and more similar to in vivo measurements.23 In addition, coculture of hiPSC-BMECs, hiPSC-astrocytes, neurons, and PCs exhibited the functional BBB efflux and high TEER value. Upregulation of tight junction proteins was demonstrated through whole genome expression profiling.133
Recently, microfluidic chips that contain multiple cell types have been developed to recreate organ-level physiology on-chip, that is, organs-on-chips or microphysiological systems.134 For example, primary human endothelial cells were cocultured with hESC-derived PCs in microfluidic channels to introduce fluid flow (also shear stress) and better recapitulate the 3D structures that have close interactions between PCs and endothelium.135 The microphysiological BBB model based on microfluidics was also recently demonstrated in simulating opioid transport after cortisol exposure.136 This model showed the decreased variability, accelerated barrier formation, and reduced passive permeability compared to transwell systems.
Human primary PCs do not reflect genetic contributions to neurodegenerative diseases and have limited scalability. To address this, hiPSC-derived BMECs, astrocytes, neurons, and brain PC-like cells were used to establish an isogenic BBB model.118 The hiPSC-derived brain PC-like cells increased TEER value (from 500 to 800 Ωcm2 up to 1156 Ωcm2) and reduced tight junction permeability and transcytosis (by 30%). Furthermore, an isogenic neurovascular unit model using four types of hiPSC-derived cells, including BMECs, brain-specific PCs, astrocytes, and neurons with retinoic acid stimulation, was reported to show enhanced functionality.137 In particular, the use of brain-like PCs significantly reduced the occurrence of nonspecific transcytosis across BMECs. The sequential PC coculture with retinoic acid-treated BMECs followed by coculture of neurons and astrocytes significantly elevated TEER value to 5486 ± 396 Ωcm2. One recent study constructed an in vitro BBB model using the hiPSC-derived BMECs, PCs, and astrocytes encapsulated in 3D Matrigel and showed that APOE4-associated cerebral amyloid angiopathy can be induced by the dysregulation of APOE and calcineurin-nuclear factor of activated T-cell signaling in the derived PC-like cells.138 But to date, the disease modeling capacity of hiPSC-based BBB models using hiPSC-PCs has not been well studied.
Conclusions
The inherent heterogeneity of brain PCs positions them to play critical roles in a wide array of neurovascular functions such as regulating BBB permeability, cerebral blood flow, and helping to facilitate the clearance of toxic cellular molecules. As a result, engineering brain-specific PCs from hiPSCs (through mesoderm induction or neural crest induction) is critical in neurodegenerative disease modeling. Studies into PCs have given insights into key signaling pathways and interactions with surrounding cells. Key pathways (PDGF-BB, TGF-β, and Notch signaling) during the interactions of PCs, endothelial cells, astrocytes, and neurons control neurovascular functions. More specifically, PDGF-BB-PDGFRβ signaling was found to promote PC survival, TGF-β signaling is involved in PC attachment to endothelial cells, and Notch signaling is critical in vascular development and arterial-venous specification. Further investigations into PC and surrounding cell interactions have shed light on PC's role in shaping neurological structure and function. The role of brain PCs in neurodegenerative disorders such as Alzheimer's disease remains unknown and their inclusion in hiPSC-derived models could prove insightful. Moving forward, gaps need to be filled in our knowledge of hiPSC-derived brain PCs in immunomodulatory abilities, major signaling pathways, ability to mediate neuroinflammation, and secretome characterizations.
Author Contributions
R.J. did the literature review and wrote initial draft of the article. J.A. did the literature review and wrote part of the article. M.M. helped the literature review and performed pericyte differentiation. J.B. helped for figure preparation. Y.L. conceived the projects, did the literature review, and wrote and revised the article.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure Statement
No competing financial interests exist.
Funding Information
This work is supported by National Science Foundation Career Award (No. 1652992 to Y.L.) and also partially supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (United States of America) under Award Number R03NS102640 (to Y.L.).
References
- 1. Hartmann D.A., Underly R.G., Grant R.I., et al. . Pericyte structure and distribution in the cerebral cortex revealed by high-resolution imaging of transgenic mice. Neurophotonics 2, 041402, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Geevarghese A., and Herman I.M.. Pericyte-endothelial crosstalk: implications and opportunities for advanced cellular therapies. Transl Res 163, 296, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Attwell D., Mishra A., Hall C.N., et al. . What is a pericyte? J Cereb Blood Flow Metab 36, 451, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rustenhoven J., Jansson D., Smyth L.C., et al. . Brain pericytes as mediators of neuroinflammation. Trends Pharmacol Sci 38, 291, 2017 [DOI] [PubMed] [Google Scholar]
- 5. Armulik A., Genove G., and Betsholtz C.. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21, 193, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Appaix F., Nissou M.F., van der Sanden B., et al. . Brain mesenchymal stem cells: the other stem cells of the brain? World J Stem Cells 6, 134, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Caplan A.I. All MSCs are pericytes? Cell Stem Cell 3, 229, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Caplan A.I. New MSC: MSCs as pericytes are sentinels and gatekeepers. J Orthop Res 35, 1151, 2017 [DOI] [PubMed] [Google Scholar]
- 9. Guimaraes-Camboa N., Cattaneo P., Sun Y., et al. . Pericytes of multiple organs do not behave as mesenchymal stem cells in vivo. Cell Stem Cell 20, 345.e5, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Crisan M., Yap S., Casteilla L., et al. . A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3, 301, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Sweeney M.D., Ayyadurai S., and Zlokovic B.V.. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci 19, 771, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Uemura M.T., Maki T., Ihara M., et al. . Brain microvascular pericytes in vascular cognitive impairment and dementia. Front Aging Neurosci 12, 80, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Winkler E.A., Bell R.D., and Zlokovic B.V.. Central nervous system pericytes in health and disease. Nat Neurosci 14, 1398, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brown L.S., Foster C.G., Courtney J.M., et al. . Pericytes and neurovascular function in the healthy and diseased brain. Front Cell Neurosci 13, 282, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Etchevers H.C., Vincent C., Le Douarin N.M., et al. . The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development 128, 1059, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Kurz H. Cell lineages and early patterns of embryonic CNS vascularization. Cell Adh Migr 3, 205, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Korn J., Christ B., and Kurz H.. Neuroectodermal origin of brain pericytes and vascular smooth muscle cells. J Comp Neurol 442, 78, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Nyul-Toth A., Suciu M., Molnar J., et al. . Differences in the molecular structure of the blood–brain barrier in the cerebral cortex and white matter: an in silico, in vitro, and ex vivo study. Am J Physiol Heart Circ Physiol 310, H1702, 2016 [DOI] [PubMed] [Google Scholar]
- 19. Wilhelm I., Nyul-Toth A., Suciu M., et al. . Heterogeneity of the blood–brain barrier. Tissue Barriers 4, e1143544, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Park T.I., Feisst V., Brooks A.E., et al. . Cultured pericytes from human brain show phenotypic and functional differences associated with differential CD90 expression. Sci Rep 6, 26587, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Daneman R., and Prat A.. The blood–brain barrier. Cold Spring Harb Perspect Biol 7, a020412, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yamazaki Y., and Kanekiyo T.. Blood–brain barrier dysfunction and the pathogenesis of Alzheimer's disease. Int J Mol Sci 18, pii: , 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Campisi M., Shin Y., Osaki T., et al. . 3D self-organized microvascular model of the human blood–brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials 180, 117, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gastfriend B.D., Palecek S.P., and Shusta E.V.. Modeling the blood–brain barrier: beyond the endothelial cells. Curr Opin Biomed Eng 5, 6, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lauschke K., Frederiksen L., and Hall V.J.. Paving the way toward complex blood–brain barrier models using pluripotent stem cells. Stem Cells Dev 26, 857, 2017 [DOI] [PubMed] [Google Scholar]
- 26. Luissint A.C., Artus C., Glacial F., et al. . Tight junctions at the blood brain barrier: physiological architecture and disease-associated dysregulation. Fluids Barriers CNS 9, 23, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Greene C., Hanley N., and Campbell M.. Claudin-5: gatekeeper of neurological function. Fluids Barriers CNS 16, 3, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bell R.D., Winkler E.A., and Sagare A.P., et al. . Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68, 409, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Montagne A., Nikolakopoulou A.M., Zhao Z., et al. . Pericyte degeneration causes white matter dysfunction in the mouse central nervous system. Nat Med 24, 326, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30. Nikolakopoulou A.M., Montagne A., Kisler K., et al. . Pericyte loss leads to circulatory failure and pleiotrophin depletion causing neuron loss. Nat Neurosci 22, 1089, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Park T.E., Mustafaoglu N., Herland A., et al. . Hypoxia-enhanced blood–brain barrier chip recapitulates human barrier function and shuttling of drugs and antibodies. Nat Commun 10, 2621, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Song L., Yan Y., Marzano M., et al. . Studying heterotypic cell-cell interactions in human brain using pluripotent stem cell models for neurodegeneration. Cells 8, 299, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim T.H., Kim S.H., Leong K.W., et al. . Nanografted substrata and triculture of human pericytes, fibroblasts, and endothelial cells for studying the effects on angiogenesis. Tissue Eng Part A 22, 698, 2016 [DOI] [PubMed] [Google Scholar]
- 34. Hollmann E.K., Bailey A.K., Potharazu A.V., et al. . Accelerated differentiation of human induced pluripotent stem cells to blood–brain barrier endothelial cells. Fluids Barriers CNS 14, 9, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lippmann E.S., Azarin S.M., Kay J.E., et al. . Derivation of blood–brain barrier endothelial cells from human pluripotent stem cells. Nat Biotechnol 30, 783, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee S.W.L., Campisi M., Osaki T., et al. . Modeling nanocarrier transport across a 3D in vitro human blood–brain-barrier microvasculature. Adv Healthc Mater 2020, e1901486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Appelt-Menzel A., Cubukova A., Gunther K., et al. . Establishment of a human blood–brain barrier co-culture model mimicking the neurovascular unit using induced pluri- and multipotent stem cells. Stem Cell Rep 8, 894, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nzou G., Wicks R.T., Wicks E.E., et al. . Human cortex spheroid with a functional blood brain barrier for high-throughput neurotoxicity screening and disease modeling. Sci Rep 8, 7413, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Katt M.E., Linville R.M., Mayo L.N., et al. . Functional brain-specific microvessels from iPSC-derived human brain microvascular endothelial cells: the role of matrix composition on monolayer formation. Fluids Barriers CNS 15, 7, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Linville R.M., DeStefano J.G., Sklar M.B., et al. . Human iPSC-derived blood–brain barrier microvessels: validation of barrier function and endothelial cell behavior. Biomaterials 190–191, 24, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jamieson J.J., Linville R.M., Ding Y.Y., et al. . Role of iPSC-derived pericytes on barrier function of iPSC-derived brain microvascular endothelial cells in 2D and 3D. Fluids Barriers CNS 16, 15, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Song L., Yuan X., Jones Z., et al. . Assembly of human stem cell-derived cortical spheroids and vascular spheroids to model 3-D brain-like tissues. Sci Rep 9, 5977, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bergmann S., Lawler S.E., Qu Y., et al. . Blood–brain-barrier organoids for investigating the permeability of CNS therapeutics. Nat Protoc 13, 2827, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Smyth L.C.D., Rustenhoven J., Scotter E.L., et al. . Markers for human brain pericytes and smooth muscle cells. J Chem Neuroanat 92, 48, 2018 [DOI] [PubMed] [Google Scholar]
- 45. Mitchell T.S., Bradley J., Robinson G.S., et al. . RGS5 expression is a quantitative measure of pericyte coverage of blood vessels. Angiogenesis 11, 141, 2008 [DOI] [PubMed] [Google Scholar]
- 46. Vanlandewijck M., He L., Mae M.A., et al. . A molecular atlas of cell types and zonation in the brain vasculature. Nature 554, 475, 2018 [DOI] [PubMed] [Google Scholar]
- 47. Reyahi A., Nik A.M., Ghiami M., et al. . Foxf2 is required for brain pericyte differentiation and development and maintenance of the blood–brain barrier. Dev Cell 34, 19, 2015 [DOI] [PubMed] [Google Scholar]
- 48. He L., Vanlandewijck M., Raschperger E., et al. . Analysis of the brain mural cell transcriptome. Sci Rep 6, 35108, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hosaka K., Yang Y., Seki T., et al. . Tumour PDGF-BB expression levels determine dual effects of anti-PDGF drugs on vascular remodelling and metastasis. Nat Commun 4, 2129, 2013 [DOI] [PubMed] [Google Scholar]
- 50. Jansson D., Scotter E.L., Rustenhoven J., et al. . Interferon-gamma blocks signalling through PDGFRbeta in human brain pericytes. J Neuroinflammation 13, 249, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rustenhoven J., Aalderink M., Scotter E.L., et al. . TGF-beta1 regulates human brain pericyte inflammatory processes involved in neurovasculature function. J Neuroinflammation 13, 37, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hill J., Rom S., Ramirez S.H., et al. . Emerging roles of pericytes in the regulation of the neurovascular unit in health and disease. J Neuroimmune Pharmacol 9, 591, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kovall R.A., Gebelein B., Sprinzak D., et al. . The canonical notch signaling pathway: structural and biochemical insights into shape, sugar, and force. Dev Cell 41, 228, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bejoy J., Bijonowski B., Marzano M., et al. . Wnt-Notch signaling interactions during neural and astroglial tissue patterning of human induced pluripotent stem cells. Tissue Eng Part A 26, 419, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Armulik A., Genove G., Mae M., et al. . Pericytes regulate the blood–brain barrier. Nature 468, 557, 2010 [DOI] [PubMed] [Google Scholar]
- 56. Winkler E.A., Sagare A.P., and Zlokovic B.V.. The pericyte: a forgotten cell type with important implications for Alzheimer's disease? Brain Pathol 24, 371, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Berthiaume A.A., Hartmann D.A., Majesky M.W., et al. . Pericyte structural remodeling in cerebrovascular health and homeostasis. Front Aging Neurosci 10, 210, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Daneman R., Zhou L., Kebede A.A., et al. . Pericytes are required for blood–brain barrier integrity during embryogenesis. Nature 468, 562, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Herland A., van der Meer A.D., FitzGerald E.A., et al. . Distinct contributions of astrocytes and pericytes to neuroinflammation identified in a 3D human blood–brain barrier on a chip. PLoS One 11, e0150360, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhao J., Davis M.D., Martens Y.A., et al. . APOE epsilon4/epsilon4 diminishes neurotrophic function of human iPSC-derived astrocytes. Hum Mol Genet 26, 2690, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Halliday M.R., Rege S.V., Ma Q., et al. . Accelerated pericyte degeneration and blood–brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer's disease. J Cereb Blood Flow Metab 36, 216, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mishra A., Reynolds J.P., Chen Y., et al. . Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles. Nat Neurosci 19, 1619, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. MacVicar B.A., and Newman E.A.. Astrocyte regulation of blood flow in the brain. Cold Spring Harb Perspect Biol 7, pii: , 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kisler K., Nelson A.R., Rege S.V., et al. . Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nat Neurosci 20, 406, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Duan L., Zhang X.D., Miao W.Y., et al. . PDGFRbeta cells rapidly relay inflammatory signal from the circulatory system to neurons via chemokine CCL2. Neuron 100, 183.e8, 2018 [DOI] [PubMed] [Google Scholar]
- 66. Marie C., Pedard M., Quirie A., et al. . Brain-derived neurotrophic factor secreted by the cerebral endothelium: a new actor of brain function? J Cereb Blood Flow Metab 38, 935, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Takahashi H., Takata F., Matsumoto J., et al. . Brain pericyte-derived soluble factors enhance insulin sensitivity in GT1–7 hypothalamic neurons. Biochem Biophys Res Commun 457, 532, 2015 [DOI] [PubMed] [Google Scholar]
- 68. Hamilton N.B., Attwell D., and Hall C.N.. Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front Neuroenergetics 2, 5, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rafalski V.A., Merlini M., and Akassoglou K.. Pericytes: the brain's very first responders? Neuron 100, 11, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Peppiatt C.M., Howarth C., Mobbs P., et al. . Bidirectional control of CNS capillary diameter by pericytes. Nature 443, 700, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nwadozi E., Rudnicki M., and Haas T.L.. Metabolic coordination of pericyte phenotypes: therapeutic implications. Front Cell Dev Biol 8, 77, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bejoy J., Yuan X., Song L., et al. . Genomics analysis of metabolic pathways of human stem cell-derived microglia-like cells and the integrated cortical spheroids. Stem Cells Int 2019, 2382534, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Liu Y., Yuan X., Munoz N., et al. . Commitment to aerobic glycolysis sustains immunosuppression of human mesenchymal stem cells. Stem Cells Transl Med 8, 93, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yuan X., Logan T.M., and Ma T.. Metabolism in human mesenchymal stromal cells: a missing link between hMSC biomanufacturing and therapy? Front Immunol 10, 977, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yuan X., Liu Y., Bijonowski B., et al. NAD+/NADH redox cycle and metabolic reconfigurations rejuvenate human mesenchymal stem cells with replicative senescence in vitro. [Epub ahead of print]; Commun Biol 2020 [DOI] [PMC free article] [PubMed]
- 76. Liu Y., Munoz N., Bunnell B.A., et al. . Density-dependent metabolic heterogeneity in human mesenchymal stem cells. Stem Cells 33, 3368, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Liu Y., Munoz N., Tsai A.C., et al. . Metabolic reconfiguration supports reacquisition of primitive phenotype in human mesenchymal stem cell aggregates. Stem Cells 35, 398, 2017 [DOI] [PubMed] [Google Scholar]
- 78. Bayin N.S., Frenster J.D., Sen R., et al. . Notch signaling regulates metabolic heterogeneity in glioblastoma stem cells. Oncotarget 8, 64932, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Xiao Y., Peng H., Hong C., et al. . PDGF promotes the Warburg Effect in pulmonary arterial smooth muscle cells via activation of the PI3K/AKT/mTOR/HIF-1alpha signaling pathway. Cell Physiol Biochem 42, 1603, 2017 [DOI] [PubMed] [Google Scholar]
- 80. Smyth L.C.D., Rustenhoven J., Park T.I., et al. . Unique and shared inflammatory profiles of human brain endothelia and pericytes. J Neuroinflammation 15, 138, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hurtado-Alvarado G., Cabanas-Morales A.M., and Gomez-Gonzalez B.. Pericytes: brain-immune interface modulators. Front Integr Neurosci 7, 80, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rudziak P., Ellis C.G., and Kowalewska P.M.. Role and molecular mechanisms of pericytes in regulation of leukocyte diapedesis in inflamed tissues. Mediators Inflamm 2019, 4123605, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cuzzocrea S., and Salvemini D.. Molecular mechanisms involved in the reciprocal regulation of cyclooxygenase and nitric oxide synthase enzymes. Kidney Int 71, 290, 2007 [DOI] [PubMed] [Google Scholar]
- 84. Wilhelm I., Nyul-Toth A., Kozma M., et al. . Role of pattern recognition receptors of the neurovascular unit in inflamm-aging. Am J Physiol Heart Circ Physiol 313, H1000, 2017 [DOI] [PubMed] [Google Scholar]
- 85. Rustenhoven J., Scotter E.L., Jansson D., et al. . An anti-inflammatory role for C/EBPdelta in human brain pericytes. Sci Rep 5, 12132, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sweeney M.D., Sagare A.P., and Zlokovic B.V.. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol 14, 133, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Davalos D., Ryu J.K., Merlini M., et al. . Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat Commun 3, 1227, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Tjakra M., Wang Y., Vania V., et al. . Overview of crosstalk between multiple factor of transcytosis in blood brain barrier. Front Neurosci 13, 1436, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Liebner S., Dijkhuizen R.M., Reiss Y., et al. . Functional morphology of the blood–brain barrier in health and disease. Acta Neuropathol 135, 311, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Geranmayeh M.H., Rahbarghazi R., and Farhoudi M.. Targeting pericytes for neurovascular regeneration. Cell Commun Signal 17, 26, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sagare A.P., Sweeney M.D., Makshanoff J., et al. . Shedding of soluble platelet-derived growth factor receptor-beta from human brain pericytes. Neurosci Lett 607, 97, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Navarro R., Compte M., Alvarez-Vallina L., et al. . Immune regulation by pericytes: modulating innate and adaptive immunity. Front Immunol 7, 480, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Orihuela R., McPherson C.A., and Harry G.J.. Microglial M1/M2 polarization and metabolic states. Br J Pharmacol 173, 649, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Chen P., Zhao W., Guo Y., et al. . CX3CL1/CX3CR1 in Alzheimer's disease: a target for neuroprotection. Biomed Res Int 2016, 8090918, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sakuma R., Kawahara M., Nakano-Doi A., et al. . Brain pericytes serve as microglia-generating multipotent vascular stem cells following ischemic stroke. J Neuroinflammation 13, 57, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kanekiyo T., and Bu G.. The low-density lipoprotein receptor-related protein 1 and amyloid-beta clearance in Alzheimer's disease. Front Aging Neurosci 6, 93, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ma Q., Zhao Z., Sagare A.P., et al. . Blood–brain barrier-associated pericytes internalize and clear aggregated amyloid-beta42 by LRP1-dependent apolipoprotein E isoform-specific mechanism. Mol Neurodegener 13, 57, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ozen I., Deierborg T., Miharada K., et al. . Brain pericytes acquire a microglial phenotype after stroke. Acta Neuropathol 128, 381, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Pober J.S., Merola J., Liu R., et al. . Antigen presentation by vascular cells. Front Immunol 8, 1907, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. ElAli A., Theriault P., and Rivest S.. The role of pericytes in neurovascular unit remodeling in brain disorders. Int J Mol Sci 15, 6453, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Xu J., Gong T., Heng B.C., et al. . A systematic review: differentiation of stem cells into functional pericytes. FASEB J 31, 1775, 2017 [DOI] [PubMed] [Google Scholar]
- 102. Greenwood-Goodwin M., Yang J., Hassanipour M., et al. . A novel lineage restricted, pericyte-like cell line isolated from human embryonic stem cells. Sci Rep 6, 24403, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Dar A., Domev H., Ben-Yosef O., et al. . Multipotent vasculogenic pericytes from human pluripotent stem cells promote recovery of murine ischemic limb. Circulation 125, 87, 2012 [DOI] [PubMed] [Google Scholar]
- 104. Kusuma S., Shen Y.I., Hanjaya-Putra D., et al. . Self-organized vascular networks from human pluripotent stem cells in a synthetic matrix. Proc Natl Acad Sci U S A 110, 12601, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Yamazaki T., and Mukouyama Y.S.. Tissue specific origin, development, and pathological perspectives of pericytes. Front Cardiovasc Med 5, 78, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Orlova V.V., van den Hil F.E., Petrus-Reurer S., et al. . Generation, expansion and functional analysis of endothelial cells and pericytes derived from human pluripotent stem cells. Nat Protoc 9, 1514, 2014 [DOI] [PubMed] [Google Scholar]
- 107. Chan X.Y., Black R., Dickerman K., et al. . Three-dimensional vascular network assembly from diabetic patient-derived induced pluripotent stem cells. Arterioscler Thromb Vasc Biol 35, 2677, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wanjare M., Kusuma S., and Gerecht S.. Defining differences among perivascular cells derived from human pluripotent stem cells. Stem Cell Rep 2, 746, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Yiangou L., Grandy R.A., Osnato A., et al. . Cell cycle regulators control mesoderm specification in human pluripotent stem cells. J Biol Chem 294, 17903, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kumar A., D'Souza S.S., Moskvin O.V., et al. . Specification and diversification of pericytes and smooth muscle cells from mesenchymoangioblasts. Cell Rep 19, 1902, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Chin C.J., Li S., Corselli M., et al. . Transcriptionally and functionally distinct mesenchymal subpopulations are generated from human pluripotent stem cells. Stem Cell Rep 10, 436, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Colunga T., Hayworth M., Kress S., et al. . Human pluripotent stem cell-derived multipotent vascular progenitors of the mesothelium lineage have utility in tissue engineering and repair. Cell Rep 26, 2566.e10, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kim J.M., Hong K.S., Song W.K., et al. . Perivascular progenitor cells derived from human embryonic stem cells exhibit functional characteristics of pericytes and improve the retinal vasculature in a rodent model of diabetic retinopathy. Stem Cells Transl Med 5, 1268, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Menendez L., Kulik M.J., Page A.T., et al. . Directed differentiation of human pluripotent cells to neural crest stem cells. Nat Protoc 8, 203, 2013 [DOI] [PubMed] [Google Scholar]
- 115. Faal T., Phan D.T.T., Davtyan H., et al. . Induction of mesoderm and neural crest-derived pericytes from human pluripotent stem cells to study blood–brain barrier interactions. Stem Cell Rep 12, 451, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lian X., Bao X., Al-Ahmad A., et al. . Efficient differentiation of human pluripotent stem cells to endothelial progenitors via small-molecule activation of WNT signaling. Stem Cell Rep 3, 804, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Leung A.W., Murdoch B., Salem A.F., et al. . WNT/beta-catenin signaling mediates human neural crest induction via a pre-neural border intermediate. Development 143, 398, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Stebbins M.J., Gastfriend B.D., Canfield S.G., et al. . Human pluripotent stem cell-derived brain pericyte-like cells induce blood–brain barrier properties. Sci Adv 5, eaau7375, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Nguyen E.H., Dombroe M.J., Fisk D.L., et al. . Neurovascular organotypic culture models using induced pluripotent stem cells to assess adverse chemical exposure outcomes. Appl In Vitro Toxicol 5, 92, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Iendaltseva O., Orlova V.V., Mummery C.L., et al. . Fibronectin patches as anchoring points for force sensing and transmission in human induced pluripotent stem cell-derived pericytes. Stem Cell Reports [Epub ahead of print]; DOI: 10.1016/j.stemcr.2020.05.001, 2020 [DOI] [PMC free article] [PubMed]
- 121. Song L., Yuan X., Jones Z., et al. . Functionalization of brain region-specific spheroids with isogenic microglia-like cells. Sci Rep 9, 11055, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Griffin K., Bejoy J., Song L., et al. . Human stem cell-derived multicellular aggregates of forebrain astroglia respond to amyloid beta oligomers. Tissue Eng Part A 26, 527, 2020 [DOI] [PubMed] [Google Scholar]
- 123. Vatine G.D., Al-Ahmad A., Barriga B.K., et al. . Modeling psychomotor retardation using iPSCs from MCT8-deficient patients indicates a prominent role for the blood–brain barrier. Cell Stem Cell 20, 831.e5, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Bosworth A.M., Faley S.L., Bellan L.M., et al. . Modeling neurovascular disorders and therapeutic outcomes with human-induced pluripotent stem cells. Front Bioeng Biotechnol 5, 87, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Zlokovic B.V. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci 12, 723, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kirabali T., Rigotti S., Siccoli A., et al. . The amyloid-beta degradation intermediate Abeta34 is pericyte-associated and reduced in brain capillaries of patients with Alzheimer's disease. Acta Neuropathol Commun 7, 194, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Nortley R., Korte N., Izquierdo P., et al. Amyloid beta oligomers constrict human capillaries in Alzheimer's disease via signaling to pericytes. Science 365, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Tachibana M., Yamazaki Y., Liu C.C., et al. . Pericyte implantation in the brain enhances cerebral blood flow and reduces amyloid-beta pathology in amyloid model mice. Exp Neurol 300, 13, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Yan Y., Song L., Bejoy J., et al. . Modelling neurodegenerative microenvironment using cortical organoids derived from human stem cells. Tissue Eng Part A 24, 1125, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Marzano M., Bejoy J., Cheerathodi M., et al. . Differential effects of extracellular vesicles of lineage-specific human pluripotent stem cells on cellular behaviours of isogenic cortical spheroids. Cells 8, 993, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Lippmann E.S., Al-Ahmad A., Azarin S.M., et al. . A retinoic acid-enhanced, multicellular human blood–brain barrier model derived from stem cell sources. Sci Rep 4, 4160, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Patel R., Page S., and Al-Ahmad A.J.. Isogenic blood–brain barrier models based on patient-derived stem cells display inter-individual differences in cell maturation and functionality. J Neurochem 142, 74, 2017 [DOI] [PubMed] [Google Scholar]
- 133. Delsing L., Donnes P., Sanchez J., et al. . Barrier properties and transcriptome expression in human iPSC-derived models of the blood–brain barrier. Stem Cells 36, 1816, 2018 [DOI] [PubMed] [Google Scholar]
- 134. Cochrane A., Albers H.J., Passier R., et al. . Advanced in vitro models of vascular biology: human induced pluripotent stem cells and organ-on-chip technology. Adv Drug Deliv Rev 140, 68, 2018 [DOI] [PubMed] [Google Scholar]
- 135. van der Meer A.D., Orlova V.V., ten Dijke P., et al. . Three-dimensional co-cultures of human endothelial cells and embryonic stem cell-derived pericytes inside a microfluidic device. Lab Chip 13, 3562, 2013 [DOI] [PubMed] [Google Scholar]
- 136. Brown J.A., Faley S.L., Shi Y., et al. . Advances in blood–brain barrier modeling in microphysiological systems highlight critical differences in opioid transport due to cortisol exposure. Fluids Barriers CNS 17, 38, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Canfield S.G., Stebbins M.J., Faubion M.G., et al. . An isogenic neurovascular unit model comprised of human induced pluripotent stem cell-derived brain microvascular endothelial cells, pericytes, astrocytes, and neurons. Fluids Barriers CNS 16, 25, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Blanchard J.W., Bula M., Davila-Velderrain J., et al. . Reconstruction of the human blood–brain barrier in vitro reveals a pathogenic mechanism of APOE4 in pericytes. Nat Med [Epub ahead of print]; DOI: 10.1038/s41591-020-0886-4 [DOI] [PMC free article] [PubMed]
- 139. Orlova V.V., Drabsch Y., Freund C., et al. . Functionality of endothelial cells and pericytes from human pluripotent stem cells demonstrated in cultured vascular plexus and zebrafish xenografts. Arterioscler Thromb Vasc Biol 34, 177, 2014 [DOI] [PubMed] [Google Scholar]