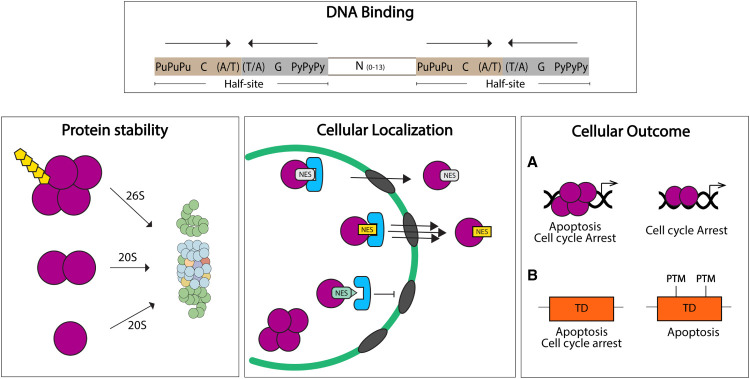
Figure 4.
The TD regulates several p53 functions. DNA Binding. The p53 response element is composed by two half-sites where two independent dimers bind and interact as a tetramer, increasing p53-DNA affinity and transactivation. Protein stability: Tetramerization is required for ubiquitin-dependent degradation of p53 (mediated by 26S proteasome). Dimers and monomers are mainly degraded by ubiquitin-independent mechanisms (20S proteasome). Cellular localization: p53 TD nuclear export signal (NES) controls p53 localization. Monomers expose the NES and retain p53 in the cytoplasm. Tetramerization allows for NES masking and nuclear localization. Mutations in the NES could regulate nuclear export (yellow and light green) by modifying affinity to the export machinery. Cellular outcome: (A) Model for oligomeric state-dependent outcomes: tetramers have full p53 activities, with the potential of initiating both apoptotic and cell cycle arrest programs. Nontetrameric p53 could have partial p53 target gene activation, favoring a certain cellular outcome (e.g., cell cycle arrest). (B) Model for TD PTM-dependent outcomes: Specific PTMs in TD residues can modulate the p53 cellular response between apoptosis and cell cycle arrest.