Figure 2.
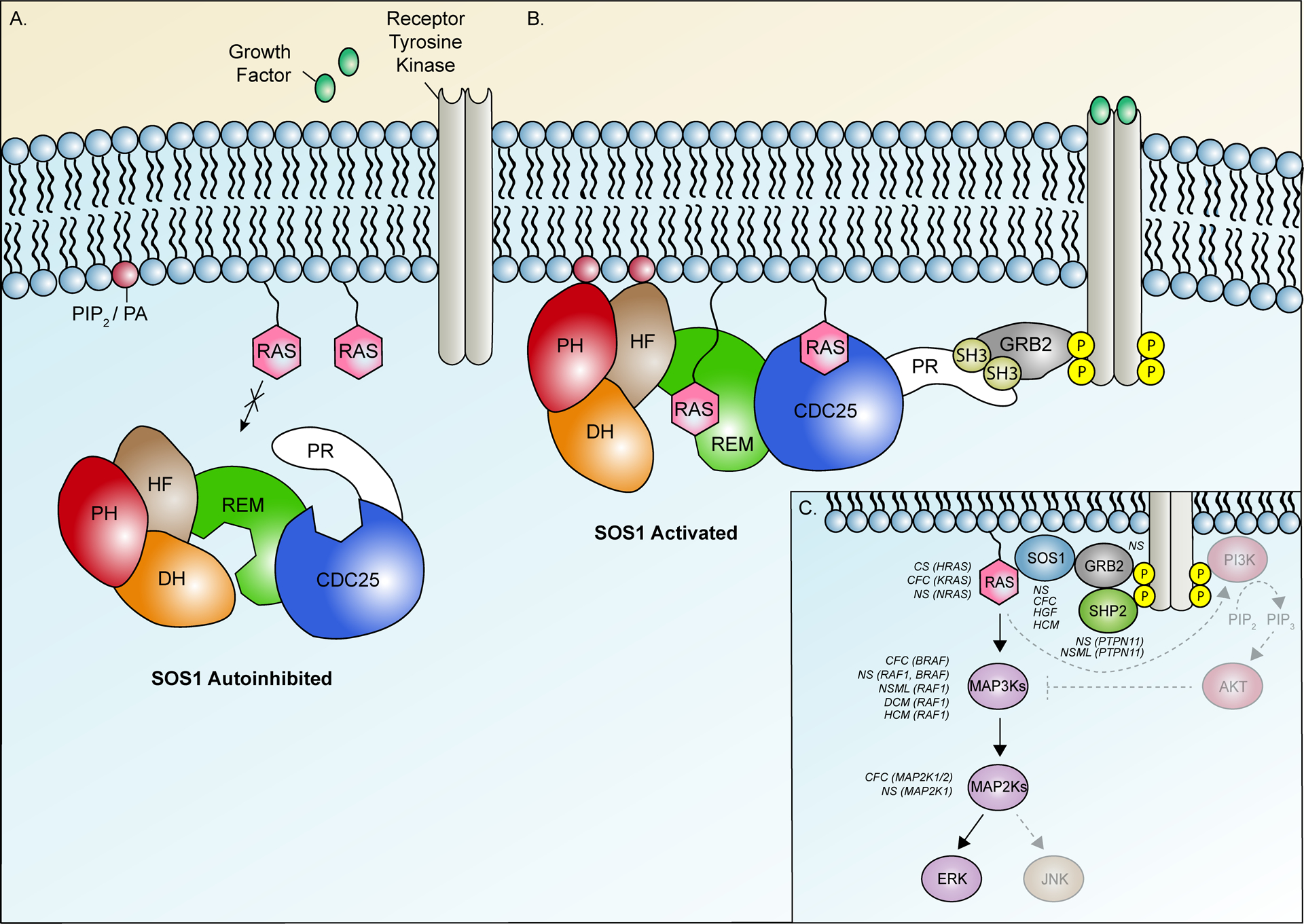
SOS1 activity is governed by complex interactions between six distinct functional domains. (A) SOS1 activity is natively suppressed by a primary auto-inhibitory unit comprised of the N-terminal histone-like fold (HF), Dbl homology (DH) domain, and pleckstrin homology (PH) domains and by secondary mechanisms facilitated by the proline-rich (PR) tail. This closed conformation blocks access of RAS to dual binding sites in the SOS1 catalytic core, comprised of the RAS-exchange motif (REM) and CDC25 domain. (B) Growth factor binding facilitates activation of receptor tyrosine kinases (RTKs) and recruitment of SOS1 to the membrane via direct interaction of the PR tail with GRB2, itself bound to phosphorylated tyrosine residues on the activated RTKs. Secondary membrane associations are further provided by the HF and PH domains via phosphatidylinositol 4,5-bisphosphate (PIP2) and phosphatidic acid (PA). Alleviation of repression by these interactions permit RAS binding to a distal binding site in the REM, initiating additional allosteric changes that recruit a second RAS molecule to the adjacent CDC25 catalytic site. Following GDP/GTP exchange, SOS1 remains strongly associated with the membrane, facilitating recruitment and activation of additional RAS molecules. (C) Simplified overview of RAS signaling pathways and associated disease phenotypes. Solid arrows represent individual steps in the ERK signaling cascade. Hatched arrows represent steps in alternate RAS-mediated AKT and JNK signaling pathways. Disease phenotypes associated with activating variants in individual pathway components are listed in italics. Affected genes are indicated in brackets. Additional abbreviations: CFC, Cardiofaciocutaneous syndrome; CS, Costello syndrome; DCM, Dilated cardiomyopathy; HCM, Hypertrophic cardiomyopathy; HGF, Hereditary gingival fibromatosis; MAP2K, Mitogen-activated protein kinase kinase; MAP3K, Mitogen-activated protein kinase kinase kinase, NS, Noonan syndrome; NSML, Noonan syndrome with multiple lentigines.
