Figure 3:
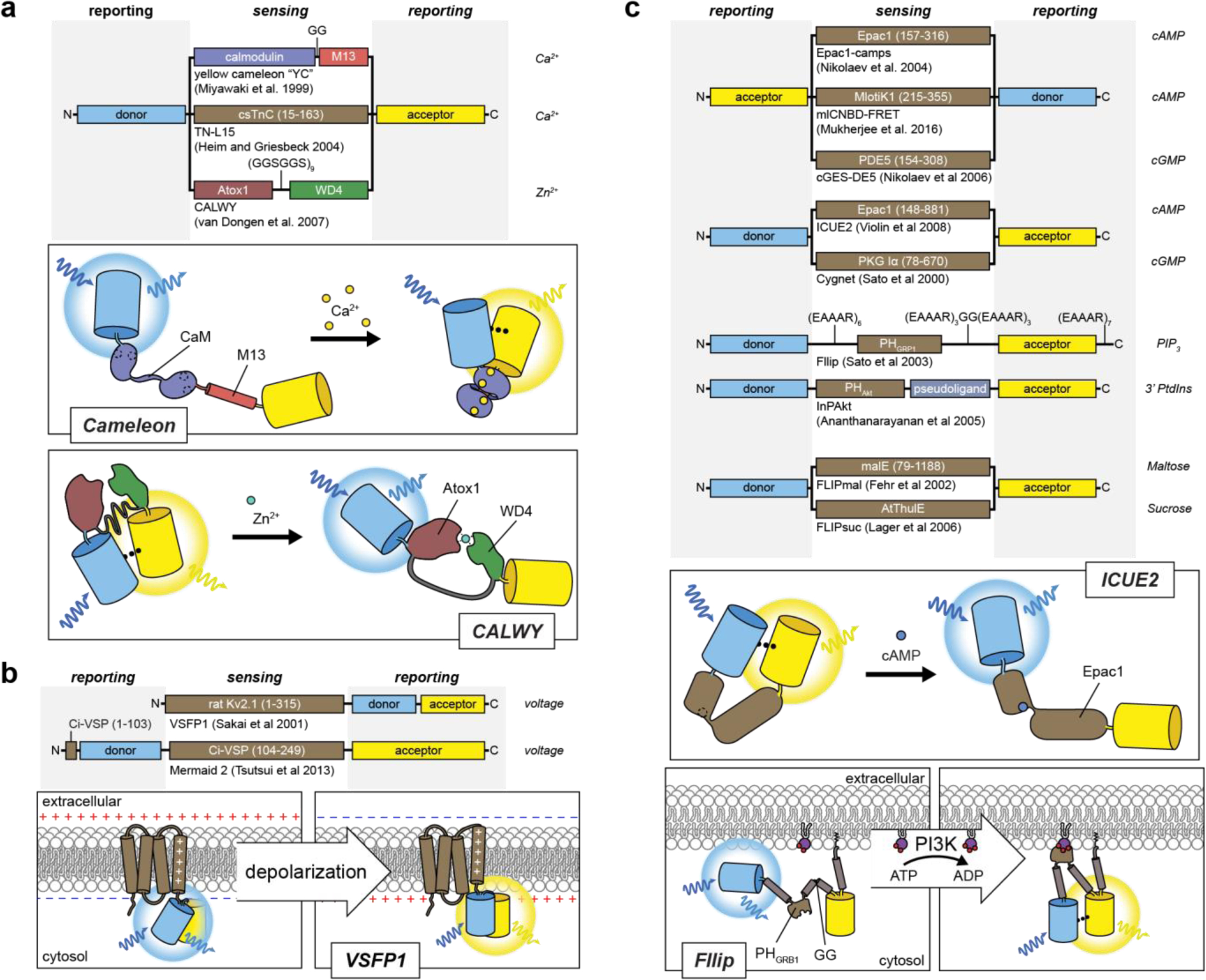
Designs of FRET-based reporters for ions, cellular analytes and membrane potential.
FRET-based metal ion biosensors utilize receiver and switch domains that bind to each other358,396, or endogenous proteins that undergo a conformational change386 in response to binding of metal ions. These conformational changes alter the intramolecular distance and orientation of a pair of FPs and thus changes the amount of energy transferred from the donor FP to the acceptor FP. B) Voltage sensors that utilize a FRET-based readout often rely primarily on changing the orientation of dipoles of FPs244,257. VSFP1 contains a CFP inserted between the 3rd and 4th transmembrane domain and a YFP fused to the C terminal tail of a truncated portion of the rat Kv2.1 channel where cell depolarization induces a conformational shift in the 4th transmembrane domain, thus changing the relative angle of the two dipoles of the fluorescent proteins257. C) Several biosensors for cellular analytes have utilized the design of sandwiching a conformationally switching domain between a FP FRET pair285,297,300,310,312,334,344,504,520. For example, the cAMP biosensor ICUE2 utilizes the conformational change induced by cAMP binding to the CNB domain of Epac1 to increase the intramolecular distance between the donor and acceptor FPs297. Alternatively, having both a PIP3 binding PH domain from GRP1 and a C-terminal membrane localization sequence in combination with engineered rigid α-helical linkers yielded a chimeric protein that exhibits significant conformational changes in response to PIP3 production, leading to changes in FRET between a FRET pair flanking the chimeric protein520.
