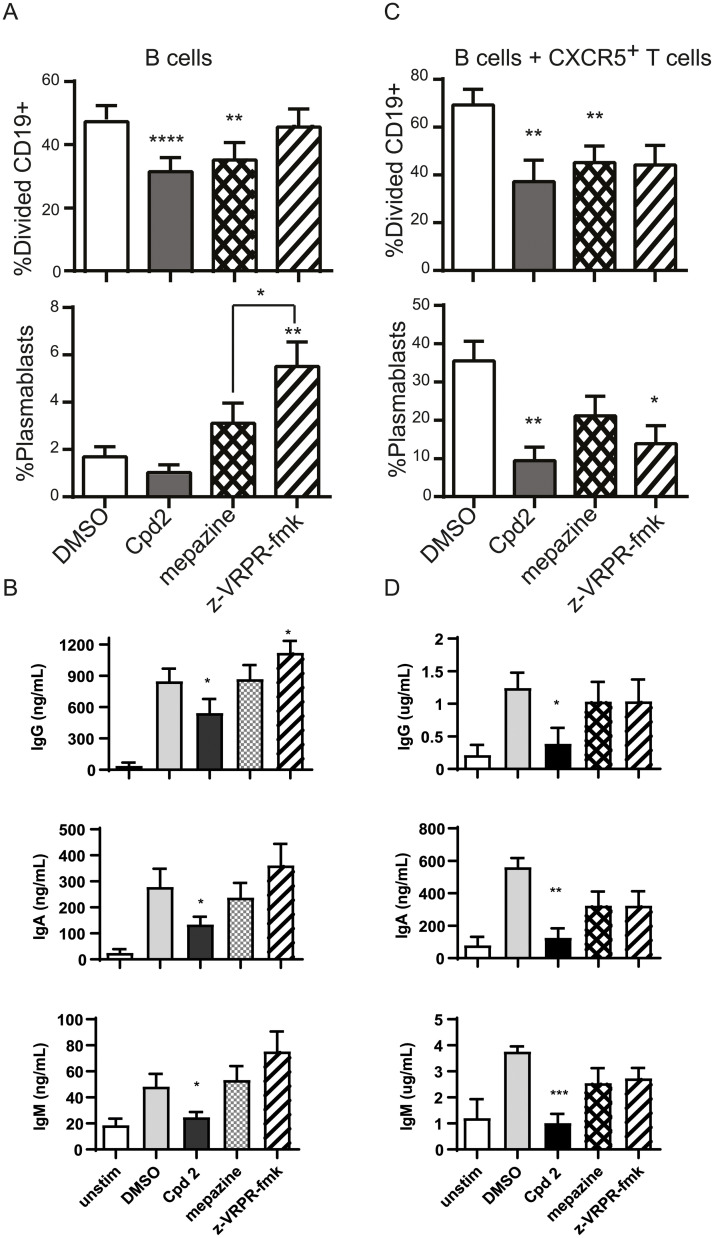
Fig 7. Allosteric MALT1 inhibitor Compound 2 reduces BCR-induced and T-cell induced B-cell immune responses.
(A) and (B) CFSE stained CD19+ cells were stimulated for 5 days with anti-IgM+anti-CD40 + IL-21 in the presence or absence of 10 μM Compound 2, 5 μM mepazine or 100 μM z-VRPR-fmk. (n = 8). Proliferation (A) was assessed as CFSE dilution of CD19+ B cells, presence of CD19+CD27hi CD38hi plasmablasts was measured using flow cytometry and (B) levels of IgG, IgA and IgM in the supernatant culture of unstimulated or stimulated B cells was measured using human MSD multiplex isotyping panel. (C) and (D) CFSE stained human CD19+ cells were co-cultured with FACS sorted CD4+CXCR5+CD45RO+ T-cells and stimulated for 5 days with SEB 100 ng/ml (n = 6) in the presence or absence of 10 μM Compound 2, 5 μM mepazine or 100 μM z-VRPR-fmk. Proliferation (A) was assessed as CFSE dilution of CD19+ B cells, presence of CD19+CD27hi CD38hi plasmablasts was measured using flow cytometry and (B) levels of IgG, IgA and IgM in the supernatant culture of unstimulated or stimulated B cells was measured using human MSD multiplex isotyping panel. Data are presented as mean ± SEM. The significance of the data was evaluated by donor-matched one-way ANOVA with Dunnett’s multiple comparison test compared to DMSO control. *:p<0.05; **:p<0.005;****:p<0.0001.