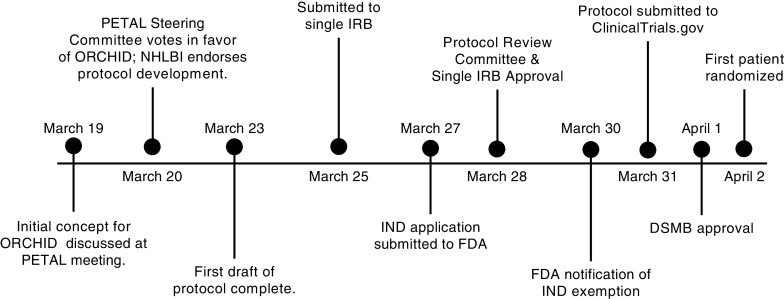
Figure 1.
Timeline from study conception to enrollment of the first patient. On February 28, 2020, the first death from coronavirus disease (COVID-19) in the United States was reported. On March 16, 2020, an initial Prevention and Early Treatment of Acute Lung Injury (PETAL) network conference call was held to discuss proposed interventions to treat COVID-19. On March 19, 2020, a brief trial concept and two-page summary was developed for a trial of hydroxychloroquine among hospitalized patients with COVID-19 and presented to the network along with other trial proposals. Following a PETAL Steering Committee vote on March 20, 2020, a trial of hydroxychloroquine was chosen as the first interventional trial for COVID-19 in the PETAL network. On the same day, the National Heart, Lung and Blood Institute reviewed the two-page summary and endorsed protocol development. A first draft of the trial protocol was completed in 72 hours and distributed to the PETAL Steering Committee. The trial protocol was finalized and submitted to the single institutional review board (IRB) on March 25, 2020. The trial was reviewed simultaneously by the single IRB and PETAL Protocol Review Committee, with both providing approval on March 28, 2020. Following an investigational new drug (IND) application submission to the Food and Drug Administration on March 27, 2020, a notification of IND exemption was received on March 30, 2020. The trial was submitted to clinicaltrials.gov on March 31, 2020. The trial was presented to the PETAL Data and Safety Monitoring Board on April 1, 2020, with approval granted on the same day. The first patient was randomized on April 2, 2020, with blinding maintained by encapsulation of hydroxychloroquine and placebo by local pharmacies. DSMB = Data and Safety Monitoring Board; FDA = Food and Drug Administration; NHLBI = National Heart, Lung, and Blood Institute; ORCHID = Outcomes Related to COVID-19 treated with Hydroxychloroquine among In-patients with symptomatic Disease.