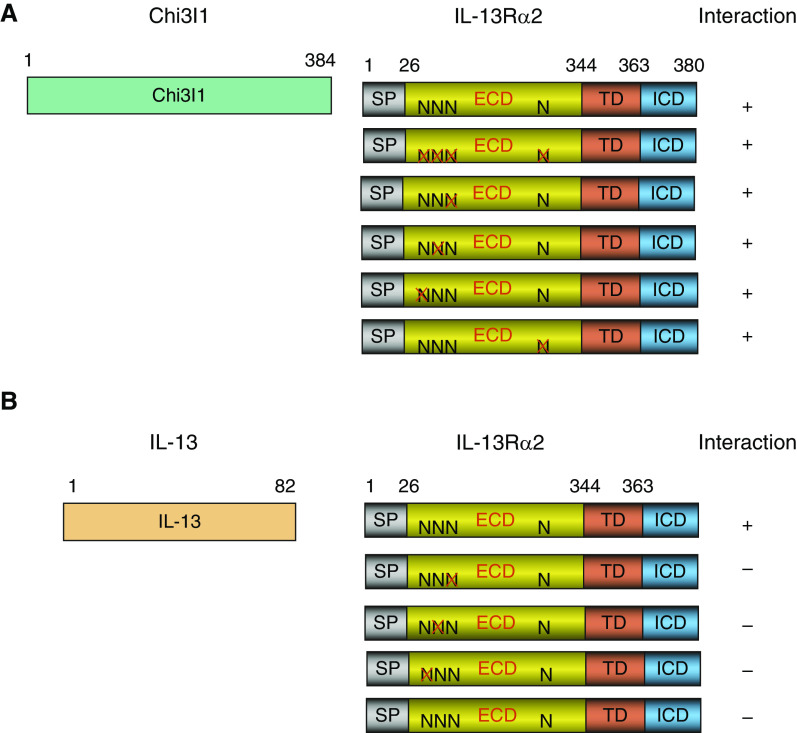
Figure 1.
Yeast 2 hybrid characterization of human (h) Chi3l1 (Chitinase 3-like-1) and IL-13 interactions with IL-13 receptor α2 (IL-13Rα2) with alterations at sites of glycosylation. (A and B) Full-length Chi3l1 (composed of 384 amino acids [A]) or IL-13 (composed of 82 amino acids [B]) was used to evaluate the interactions with full-length IL-13Rα2 (380 amino acids) composed of signal peptide (SP), extracellular domain (ECD), transmembrane domain (TD), and C-terminal intracellular domain (ICD). The predicted N-glycosylation sites (N) in ECD were individually mutated (X), and the final interactions were indicated as positive (+) or negative (−). Each panel is representative of a minimum of three evaluations.