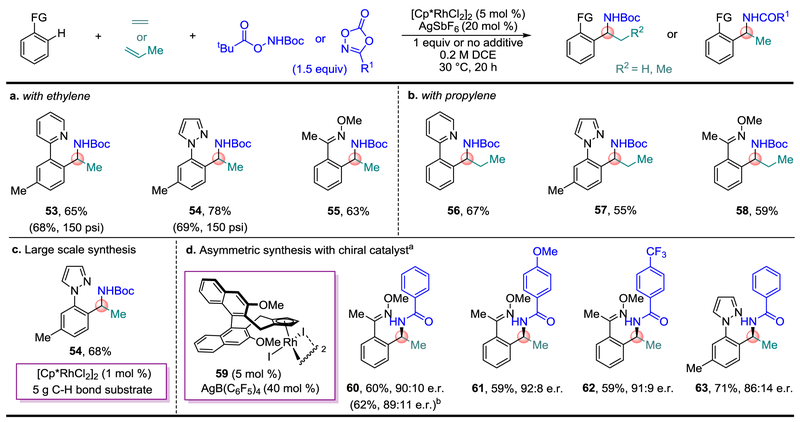
Fig. 4. Use of Feedstock Alkenes and Asymmetric Synthesis.
a and b, Synthesis of α-methyl and α-ethyl branched amines from the feedstock chemicals ethylene and propylene, respectively. c, Large scale (> 5 g) modular synthesis of Boc-protected α-methyl branched amine with 1 mol % [Cp*RhCl2]2 loading. d, Asymmetric catalysis with chiral catalyst 59. One equivalent of NaHCO3 was used as an additive for oxime C–H bond substrates. a Reactions performed on 0.05 mmol scale. b Reaction performed on 0.2 mmol scale.