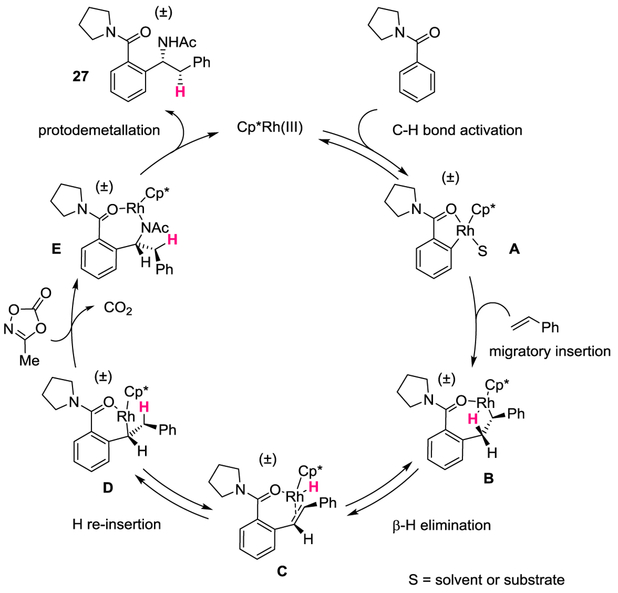
Fig. 5. Proposed catalytic cycle.
The transformation proceeds through a reversible C–H bond activation (A), followed by migratory insertion into styrene to give B. Syn β-hydride elimination and hydride re-insertion lead to the species D, which coordinates with the dioxazolone and inserts into it to give E. Protodemetallation provides the product 27 and releases the Rh(III) species to maintain catalytic turnover.