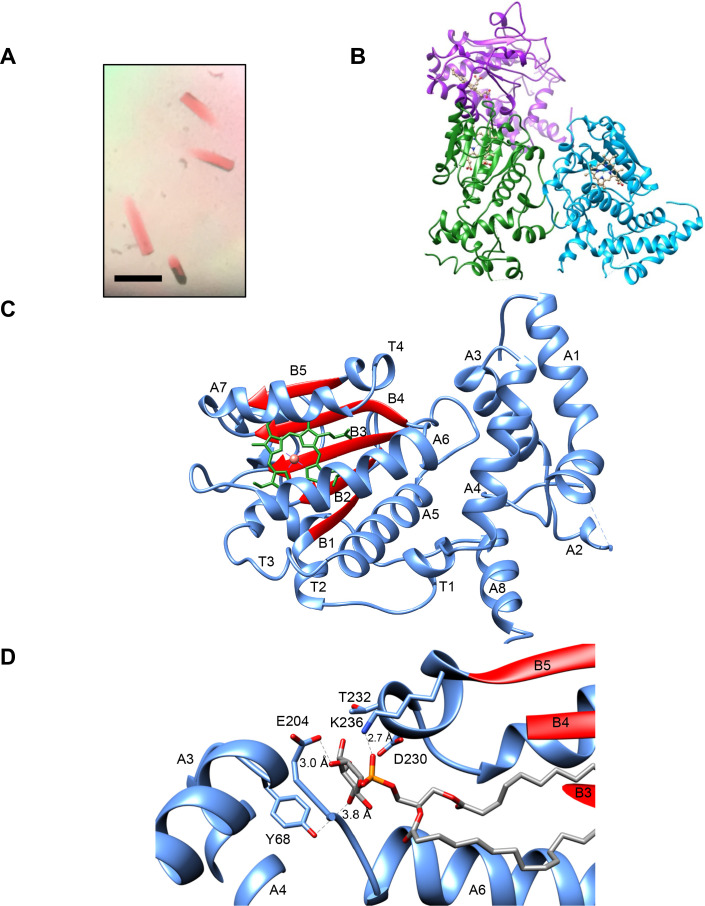
Figure 2. Sfh5 crystal structure.
(A) Recombinant Sfh5 forms reddish brown rod-shaped crystals. Scale bar, 50 µm. (B) The asymmetric unit consists of three Sfh5 molecules and ribbon representations are colored by molecule. The bound heme is shown in ball and stick with carbon atoms colored in brown, oxygen – in red, and nitrogen in blue. (C) Ribbon diagram of the Sfh5 showing α-helices, loops and 310 turns in blue, and β-strands in red. A single non-covalently-bound heme b molecule is rendered in green with the brown sphere representing the heme iron. (D) The PtdIns binding substructure of Sec14-like PITPs is conserved in Sfh5. A PtdIns molecule was modeled into the closed Sfh5 structure by overlaying Sfh1::PtdIns crystal structure (PDB ID: 3B7N) onto the Sfh5 crystal structure. This binding model emphasizes the conserved interactions (dashed lines) between Sec14 family PtdIns-binding barcode residues of Sfh5 and elements of the PtdIns headgroup. The corresponding distances between PtdIns headgroup structural elements and side-chains of the barcode residues in this dock model are shown.