Fig. 3.
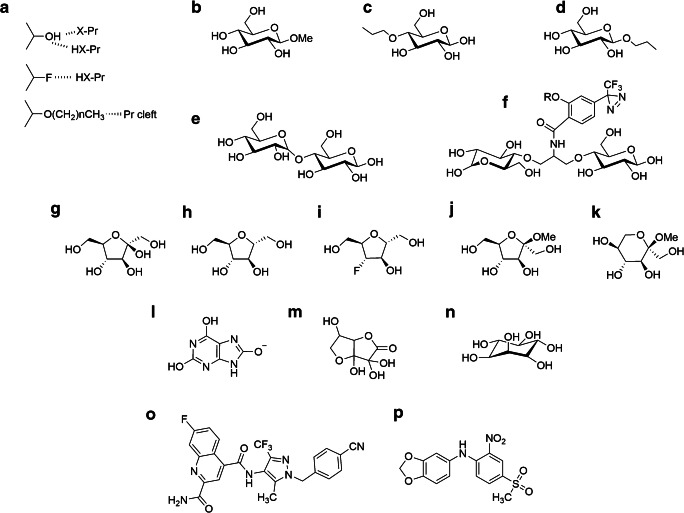
Substrates and inhibitors reveal differing specificity requirements for the GLUTs and may aid the therapeutic targeting of individual GLUTs that are implicated in disease. In a substrate and inhibitor, interactions with the GLUTs are associated with H-bonding (involving either electron donating or withdrawing groups) that can be examined using analogues that are H-bond accepting only (fluorine substitution for –OH). Spatial limitations to binding can be explored using O-alkyl groups. In b, β-methyl-D-glucoside has very low affinity for the outside site of GLUT1 suggesting a close approach to C1-O, while a 4-O-propyl group (c) is well tolerated at the outside site. The outside site can accommodate quite bulky substitutions at C4-OH with disaccharides such as maltose (e and Fig. 2 in OPO-S), and bis-glucose propylamine BGPA (f) derivatives being well tolerated. In f, the R group on the phenyl-diazirine photoreactive moiety can be a very large spacer arm with biotin. In contrast to these spatial restraints at the outside site, C1-O substitutions as in β-O-propyl-glucoside (d) or β-O-nonyl-glucoside (Fig. 2 in OPI-S) are well tolerated at the inward-facing site. Both fructofuranose and fructopyranose forms of fructose are transported by GLUTs such as GLUT5. The closed ring forms, including 2-5-anhydro-D-mannitol (h) and the β-methyl-fructofuranosides and β-methyl-fructopyranosides (j and k), are good substrates and inhibitors. Several new derivatives, including fluorescent and photolabeling compounds, based on 2-5-anhydro-D-mannitol have been described. The introduction of an H-bond accepting fluoro group at C3 of 2-5-anhydro-D-mannitol (i) reduces affinity for GLUT5 but increases affinity for GLUT1 suggesting tuning analogues for a specific GLUT is possible. The GLUT family is not restricted to glucose or fructose as substrate. GLUT9 is a transporter for urate (l). Dehydro-ascorbate in solution as a hydrate (m) is transported well by several GLUTs, and particularly GLUT10. GLUT13 transports myo-inositol (n) with good specificity. Now that GLUT structures are available, therapeutic targeting of individual GLUTs is becoming possible. In silico docking-aided screening of compound libraries has led to the identification of Bay 876 (o) as a high affinity inhibitor of GLUT1 (and not other class 1 GLUTs) and MSNBA (p) as a specific inhibitor of GLUT5 with negligible affinity for class 1 GLUTs