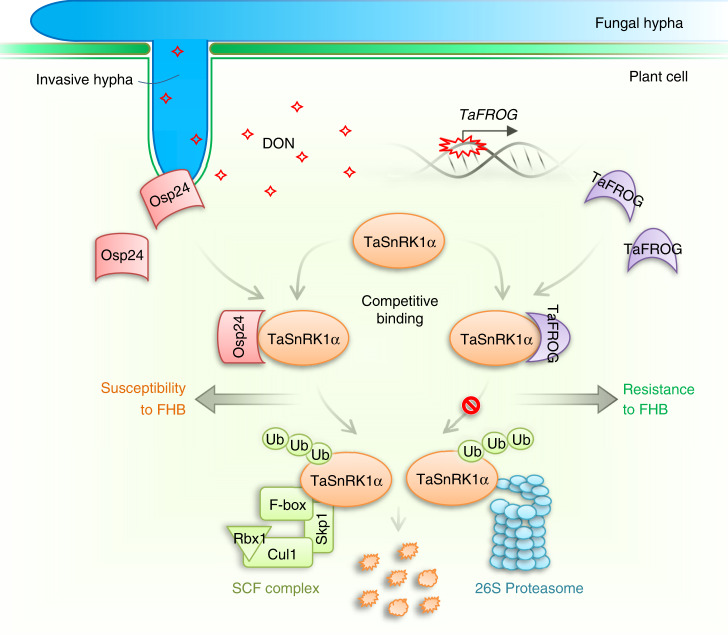
Fig. 5. A schematic summary of the roles of two orphan proteins from wheat and Fusarium graminearum during fungal–plant interactions.
The cytoplasmic effector Osp24 secreted by invasive hyphae that develop in infected wheat tissues are translocated into plant cells. It interacts with TaSnRK1α, a protein kinase activated by DON and important for resistance against F. graminearum in wheat. The association of Osp24 with TaSnRK1α stimulates its degradation by the SCF ubiquitin ligase complex and 26S proteasome in infected plant cells, resulting in increased susceptibility to Fusarium head blight (FHB). However, DON produced by the fungal pathogen during infection induces the expression of TaFROG in wheat heads. TaFROG, a Pooideae-specific orphan protein, competes with Osp24 for binding with TaSnRK1α and increases its stability. Protection by TaFROG from proteasome degradation and activation by DON enhance the functions of TaSnRK1α in regulating defense responses against F. graminearum infection.