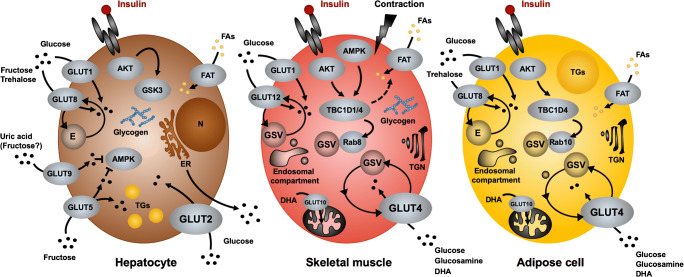
Fig. 2.
Major facilitative glucose transporters of the GLUT family in the liver, skeletal muscle, and adipose tissue. Several glucose transporters of the SLC4A2 family are involved in cellular uptake of hexoses. Entry of glucose into hepatocytes is mainly catalyzed by the low-affinity, high-capacity GLUT2 transporter which is localized on the cell surface. Following insulin stimulation, glucose is stored as glycogen or released through an ER-dependent mechanism. Other hepatic GLUTs may have accessory functions such as transporting fructose or uric acid. GLUT4 is the principal glucose transporter in adipose and muscle cells and recycles between the plasma membrane and intracellular storage vesicles. Its steady-state distribution is regulated through insulin- and/or contraction-dependent signaling cascades that involve the RabGAP proteins TBC1D1 and TBC1D4. Rab8 and Rab10 have been identified as major GTPases involved in GLUT4 translocation in muscle and fat cells, respectively. In muscle cells, GLUT12 has been described to undergo regulated traffic in response to metabolic stimuli, similar to GLUT4, whereas GLUT8 recycles in adipose cells through endosomal compartments without a known stimulus for translocation. GLUT10 has been shown to facilitate entry of oxidized vitamin C into mitochondria. At least in skeletal muscle, RabGAPs are involved in the regulated entry of fatty acids (FAs) through fatty acid transporters. Arrows indicate flow of substrates, signaling. AKT, protein kinase B; AMPK, 5′ AMP-activated protein kinase; DHA, dehydroascorbic acid; E, endosomal vesicles; ER, endoplasmic reticulum; FAT, fatty acid transporters; GSK3, glycogen synthase kinase 3; GSV, glucose transporter storage vesicles; TGN, trans-Golgi network