Table 1.
Oxidative cross-coupling using transition metal catalysts to form phosphoramidates.
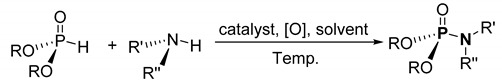
| ||||||
|---|---|---|---|---|---|---|
| Route | Catalyst | Reaction Conditions | R | R’, R’’ | Yield Range/% | Ref. |
| a | 20 mol% CuI | MeCN, 55 °C | Me, Et, i-Pr | H, alkyl | 16–98 | [103] |
| b | 5 mol% CuBr | EtOAc, 20 °C | Et, i-Pr, Bu | H, Ph, functional Ph | 20–94 | [104] |
| c | 15 mol% Fe3O4@MgO | CCl4, 20 °C | Et, i-Pr | H, Ph, Bn, cycloalkyl | 52–85 | [105] |
| d | 200 mol% CuCl2 | Acetone, Cs2CO3, 20 °C | Me, Et, Pr, i-Pr | H, alkyl | 25–93 | [106] |
| e | 10 mol% Cu(OAc)2 | Toluene, K2CO3, mol. sieve, 80 °C | Me, Et, i-Pr, Bu | RCOR a | 52–99 | [107] |
| f | 2 mol% CuBr | EtOAc, 25 °C | Me, Et, i-Pr, Bu, Ph | H, alkyl, cycloalkyl, R b | 86–96 | [108] |
| g | 5 mol% Cu(OAc)2 | MeOH, NaN3, 20 °C | Et c | R-B(OH)2 d | 67–93 | [109] |
a urea, oxazolidinone, indole, pyrrolidinone, lactam, and sulfonamide derivatives; b methyl alaninate; c triethyl phosphite; d phenylboronic acid and phenylboronic ester derivatives.
