Table 1.
Optimizing reaction conditions for imidazole synthesis a.
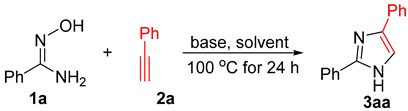
| Entry | 2a (equiv) | Base(equiv) | Solvent | Yield(%)b |
|---|---|---|---|---|
| 1 | 2.0 | Na2CO3(4) | DMSO | 10 |
| 2 | 2.0 | K2CO3(4) | DMSO | 34 |
| 3 | 2.0 | KOH(4) | DMSO | 53 |
| 4 | 2.0 | KOtBu(4) | DMSO | 41 |
| 5 | 2.0 | Cs2CO3(4) | DMSO | 75 |
| 6 | 2.0 | Cs2CO3(4) | THF | 10 |
| 7 | 2.0 | Cs2CO3(4) | Dioxane | 14 |
| 8 | 2.0 | Cs2CO3(4) | DMF | 21 |
| 9 | 1.0 | Cs2CO3(4) | DMSO | 59 |
| 10 | 1.5 | Cs2CO3(4) | DMSO | 68 |
|
11 12 13 14 |
2.0 2.0 2.0 2.0 |
Cs2CO3(2.5) Cs2CO3(1.0) Cs2CO3(0.5) -- |
DMSO DMSO DMSO DMSO |
73 33 19 0 |
a The reactions were carried out using 1a (1.0 mmol), 2a (1.0~2.0 equiv), and base in 4.0 mL of solvent in a sealed tube at 100 oC for 24 h. b yields of 3aa are isolated yields.
