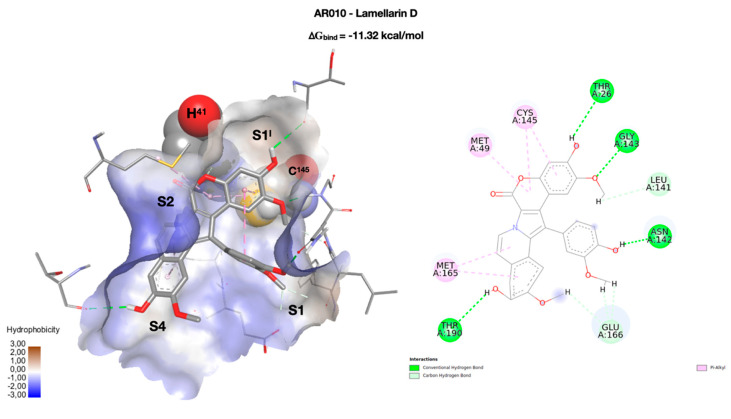
Figure 3.
On the left, the best docking conformation of lamellarin D (AR010 in stick) inside the enzymatic binding site. The binding site is represented as a solvent accessible surface (SAS) colored according to the hydrophobicity of the residues (wireframe). The two key residues of the catalytic dyad (H41 and C145) are rendered in CPK, and the four binding pockets (S1I, S1, S2, and S4) occupied by the molecule are highlighted. On the right, a two-dimensional representation of the molecular interactions between the ligand and the enzyme. The colors used refer to the different interactions, as indicated in the legend.