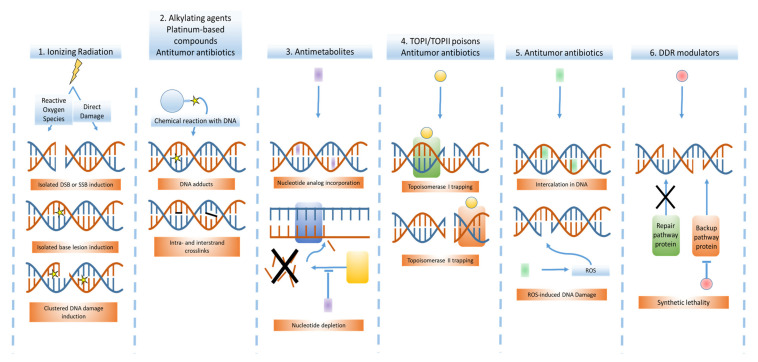
Figure 4.
Visualization of the DNA-targeted mechanisms of action of radiotherapy, cytotoxic chemotherapy and DDR modulators. 1. Ionizing radiation (IR) can induce DNA damage both directly and indirectly (through ROS formation), leading to formation of SSBs, DSBs and different base lesions. Depending on the radiation type used, clustered (complex) DNA damage might be induced when multiple DNA lesions are formed in close vicinity. 2. Alkylating and platinum-based compounds harbor a reactive site and directly react with the DNA molecule., forming DNA adducts and intra- or interstrand crosslinks. 3. Antimetabolites mimic molecules essential in DNA replication and repair and, depending on the specific compound, can be incorporated in the DNA leading to DNA damage. Alternatively. antimetabolites can inhibit nucleotide producing pathways. 4. TOPI- and TOPII-poisons, the most clinically relevant topoisomerase inhibitors, trap topoisomerases on the DNA, preventing re-ligation of topoisomerase-induced breaks. For TOP1 poisons, DSBs are formed when DNA polymerase stalls on this trapped complex. However, for TOPII-poisons, trapping leads to the persistence of topoisomerase-induced DSBs. 5. Antitumor antibiotics can have different DNA-targeted mechanisms of action, such as compound intercalation in the DNA and induction of ROS formation. Other antitumor antibiotic mechanisms of action overlap with other cytotoxic chemotherapeutic classes, such as DNA alkylation and topoisomerase poisoning. 6. DDR modulators target specific DDR proteins and exert their cancer-inhibiting effect by synthetic lethality: as cancer cells that have loss-of-function mutations in specific DDR pathways become more reliant on backup repair pathways, inhibition of the latter by DDR modulators can specifically target cancer cells versus healthy cells.