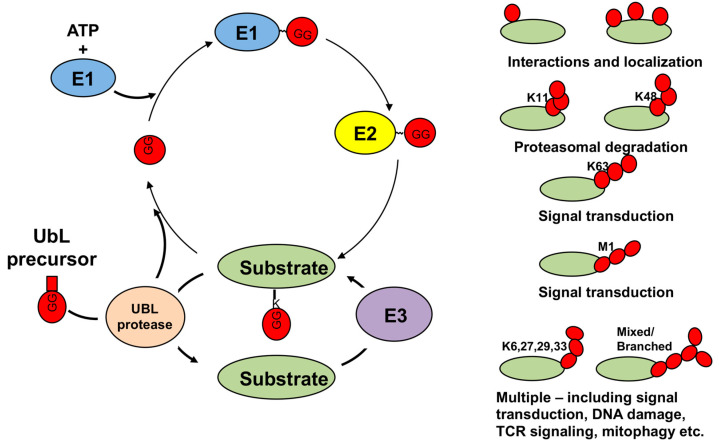
Figure 1.
Schematic illustration of the UbL activation, conjugation and deconjugation cycle. The covalent attachment of UbLs to their substrates involves sequential catalytic reactions that initiate with processing of the UbL precursor by a specific UbL protease. The mature UbL is activated by an activating enzyme (E1) and then transferred to a conjugating enzyme (E2) that, with the help of a substrate-specific ligase (E3), transfer the activated UbL to the ε-amino residue of a Lys on the target protein via a covalent isopeptide bond. Additional UbLs can be linked to the previous one to form chains. UbL-specific proteases can reverse the modification, supplementing the cellular pools of free UbLs. The attachment of a Ub moiety to the N-terminal Met1 or to an internal Lys residue of the previous Ub (K6, K11, k27,K29,K33,K48 or K63) results in the formation of topologically different poly-Ub chains that, upon recognition by signal transducers contain dedicated binding domains, target the substrates various fates and cellular functions