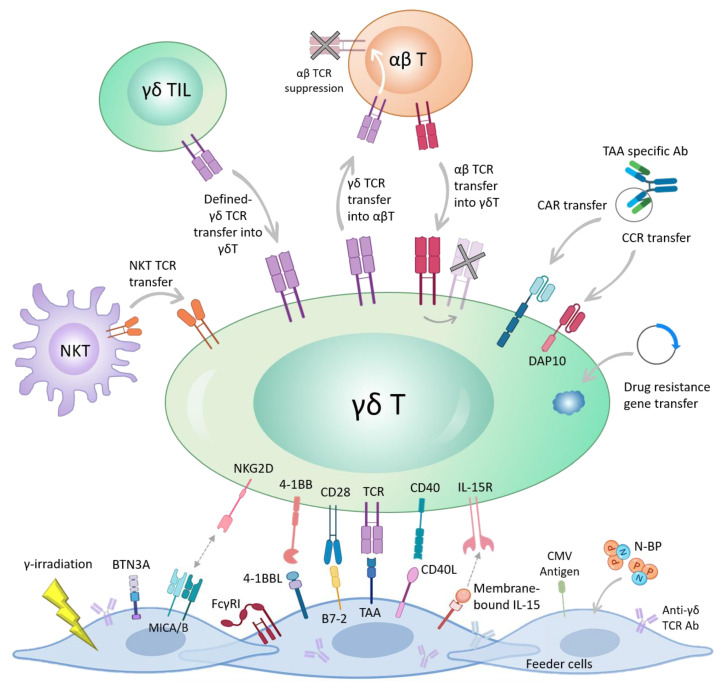
Figure 1.
Different genetic engineering strategies for harnessing the anti-tumor activity of γδ T cells. TCR-based engineering methods include TCR gene transfer from αβ to γδ T cells and vice versa. Once new TCR is introduced into αβ T cells, the expression of endogenous αβ chains will be suppressed. Synthetic CAR and chimeric co-stimulatory receptor (CCR) DNA can be transferred to γδ T cells to redirect γδ T cells toward a specific tumor antigen. CCR is similar to CAR, but lacks the CD3ζ domain, thus full activation of γδ T cells requires the support of additional co-stimulators (e.g., endogenous CD3). Transduction with drug resistance gene is another strategy aiming for rendering γδ T cells resistant to chemotherapy drugs. NKT receptor can be transferred into γδ T cells. The TCR from TILs γδ T cells may be transferred into new γδ T cells to endow anti-tumor response. Artificial APC or feeder cells may be treated with nitrogenous bisphosphonates (N-BP) to better activate γδ T cells. In addition, aAPCs may be engineered to express anti-γδ TCR Abs or different co-stimulatory molecules/ligands such as 4-1BB, B7-2, CD40L, membrane-bound IL-15 and cytomegalovirus (CMV) peptide antigens. Normally γ-irradiation is performed to hinder the aAPC growth. Stressed aAPCs, due to γ-irradiation or exposure to N-BP, up-regulate MICA/B and Butyrophilin (BTN) proteins which bind to NKG2D and activate γδ T cells. A tumor-associated antigen (TAA) may be also expressed by aAPC to selectively expand the TAA-reactive γδ T cells.