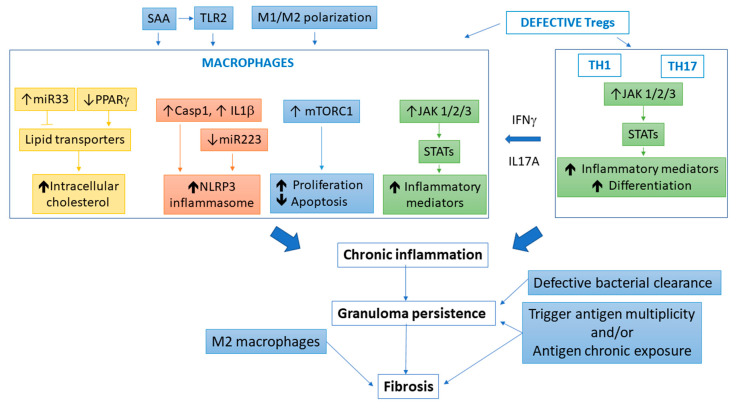
Figure 1.
Models contribution to the understanding of sarcoidosis disease progression. In sarcoidosis, an uncontrolled pro-inflammatory phenotype takes place and appears to be amplified by the activation of both extracellular (SAA, macrophage polarization, Treg deficiency) and intracellular (TLR2, lipid metabolism, NLRP3 inflammasome, mTORC1, JAK/STAT) signaling pathways. Activation of these intracellular pathways promotes a chronic inflammatory status of macrophages. The switch “chronic inflammation/granuloma persistence” is probably due to either trigger antigens multiplicity or/and antigen chronic exposure associated with a defect in pathogen clearance. Later, the switch to the M2-like phenotype may participate to initiate or amplify the process of fibrogenesis surrounding granulomas. mTORC1, mammalian/mechanistic target of rapamycin complex 1; PPAR, peroxisome proliferator-activated receptor; SAA, serum amyloid A; TLR, toll-like receptor; Casp1, caspase 1; NLRP3, NOD-like receptor (NLR) pyrin domain-containing protein 3; JAK, Janus kinase; SAA, serum amyloid A; STAT, signal transducers and activators of transcription.