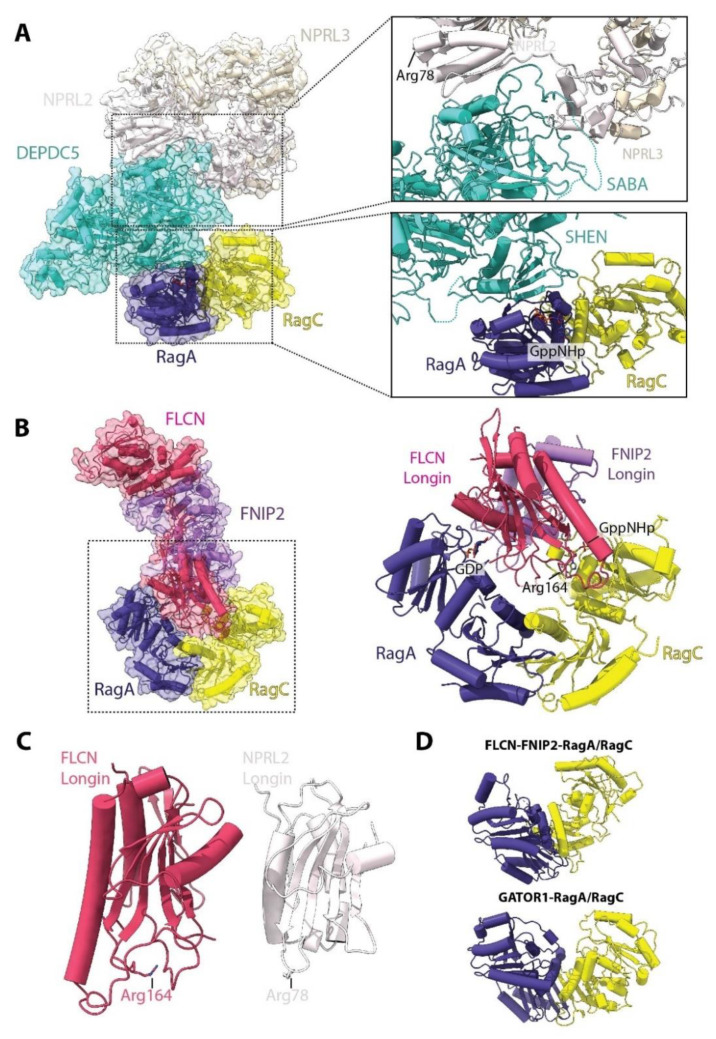
Figure 5.
Structure of the RagA and RagC GAPs GATOR1 and FLCN-FNIP2. (A) Structure of the GATOR1-RagA/RagC (PDB:6CES) complex DEPDC5 interacts with RagA via its SHEN domain and with NPRL2 via its SABA domain. The catalytic Arg78 of NPRL2 is shown mapped to the structure, being far away from the RagA nucleotide binding site. (B) Structure of the FLCN-FNIP2-RagA/RagC complex (PDB:6ULG). FLCN-FNIP2 interacts with RagA/RagC via their Longin domains. The catalytic Arg164 of FLCN is indicated and is located distant from the RagC nucleotide binding site. (C) Comparison of the Longin domains of FLCN and NPRL2, the subunits with GAP activity from FLCN-FNIP2 and GATOR1, respectively. The position of the catalytic arginine for each is shown. (D) Comparison of the conformation of RagA/RagC when bound to FLCN-FNIP2 (top) or GATOR1 (bottom). The space between GD is the widest when bound to FLCN-FNIP and similarly smaller when bound to GATOR1 or Raptor.