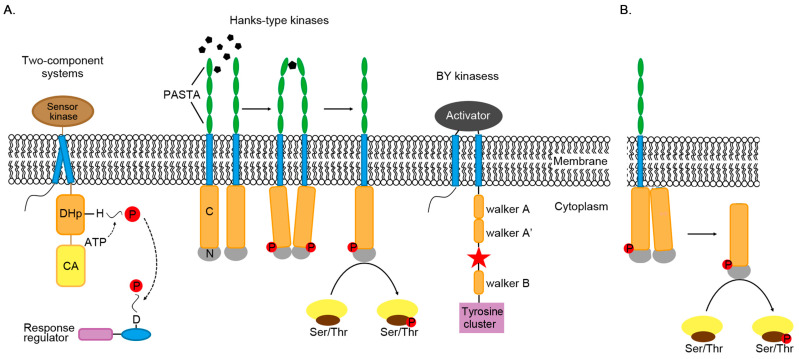
Figure 1.
Phosphorylation mechanism of three types of kinases. (A) Three types of protein kinases catalyze the transfer of phosphate groups (P) to histidine (H), aspartic acid (D), serine, threonine and tyrosine. Most of these kinases are bound to the membrane. Extracellular domain PASTA (penicillin-binding protein and serine/threonine kinase-associated) binds peptidoglycans and induces the intracellular catalytic domains closer, resulting in activating kinase domains by autophosphorylation. After phosphorylation, two monomers dimerize through the back sides of the N-terminal lobes (N). BY kinase will interact with its activator to stabilize its ATP-binding domain. The red star is the phosphorylation site. DHp is the abbreviation of dimerized histidine phosphate transfer domain, and CA is the abbreviation of catalyzed binding to ATP. (B) In STK, activated kinases can directly phosphorylate target proteins.