Figure 1. Temperature sensing by measuring membrane thickness.
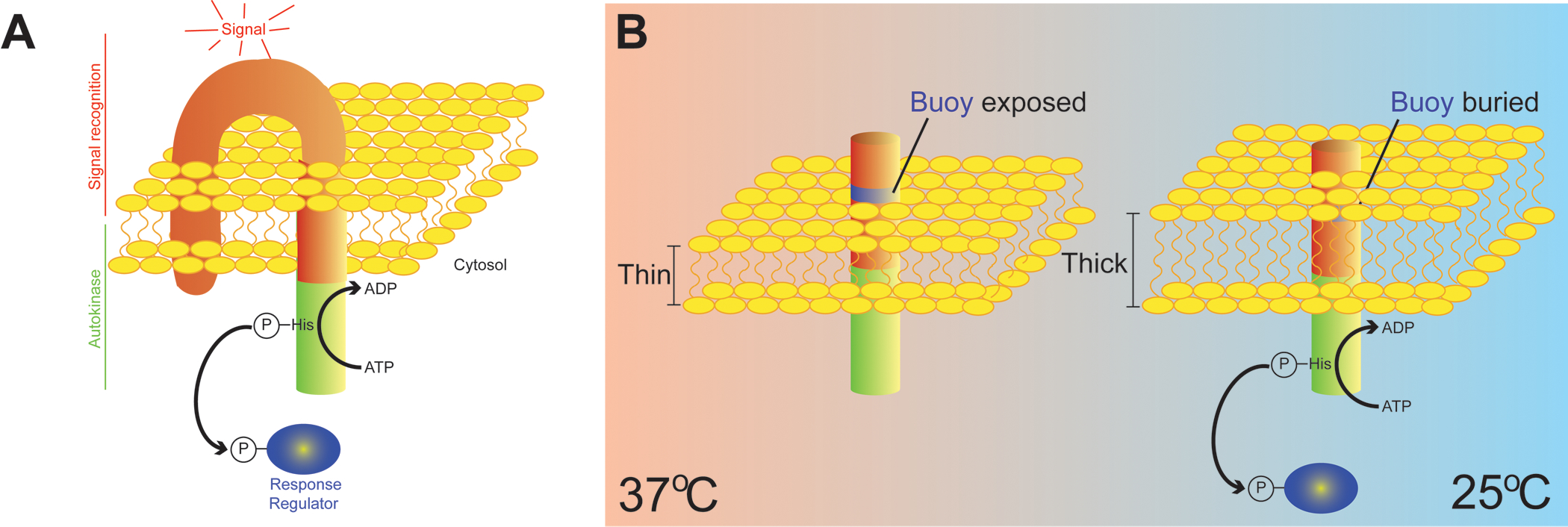
(A) Depiction of a canonical bacterial two component regulatory system. A transmembrane sensor kinase is shown with an extracytoplasmic signal recognition domain (red) and an intracellular autokinase domain (green). Sensor kinases often have multiple membrane-spanning domains, but for simplicity, only two membrane-spanning domains are depicted. Detection of an activating signal results in autophosphorylation of a characteristic histidine residue in the autokinase domain and subsequent transfer of the phosphoryl group onto a cognate response regulator (blue), typically a transcription factor. (B) Depiction of an engineered minimal signal sensing domain of DesK, a sensor kinase in Bacillus subtilis that detects changes in environmental temperature. The minimal thermo-sensor harbors a cluster of hydrophilic amino acids (blue ring) near its amino terminus (extracytoplasmic) that floats like a buoy near the lipid/water interface at high temperatures when the membrane is thinner (left). At lower temperatures (right), an increase in lipid ordering results in a thicker membrane, forcing the “buoy” into the hydrophobic lipid bilayer, thereby activating the autokinase domain. DesK therefore responds to changes in temperature by directly measuring membrane thickness.
