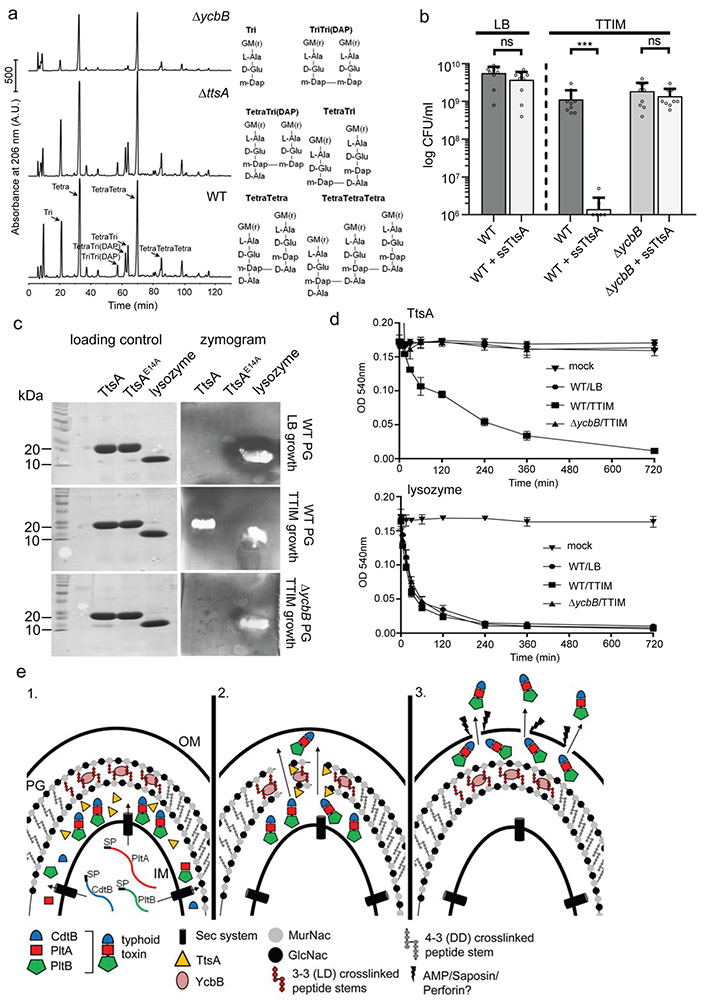
Figure 6. TtsA activity requires YcbB-mediated peptidoglycan editing.
(a) Muropeptide profiles of different S. Typhi strains showing the YcbB-dependent PG editing. The indicated S. Typhi strains were grown 24 hs in TTIM, PG was isolated and analyzed by HPLC-MS. The ycbB mutant shows a significant decrease in the monomeric disaccharide tripeptide (Tri) and the 3-3 cross-linked muropeptides TriTri(Dap) and TetraTri(Dap). (b) YcbB-dependent growth arrest after sec-dependent TtsA translocation to the periplasmic space. S. Typhi wild type and the ΔycbB isogenic mutant strain, both carrying a plasmid expressing ttsA containing a sec-secretion signal (sec-TtsA) under the control of an arabinose-inducible promoter, were grown in LB or TTIM media containing 0.001 % arabinose, and the number of CFU was determined after 24 hs of growth. Values represent the mean +/− standard deviation (*** p < 0.001, n. s. difference not statistically significant, p = 0.1367 and p = 0.316, two-sided Student’s t test). (c and d) The TtsA muramidase activity is dependent on YcbB. Peptidoglycan was isolated from wild-type S. Typhi grown in either LB, or TTIM, or from the ΔycbB S. Typhi mutant grown in TTIM, as indicated. In-gel digestion zymograms were performed with equal amounts of purified wild-type TtsA, the catalytic mutant TtsAE14A, or lysozyme (shown in a coomassie stained PAGE, left panels) (c). Alternatively, the activity of purified TtsA and lysozyme (as a control) was evaluated using a turbidimetric assay using purified peptidoglycan as indicated above (d). Graphs show the mean turbidity (measured at OD540 nm) ± standard deviation. All data in (a-d) were derived from at least three independent experiments. (e) Model for the typhoid toxin secretion mechanism. The different subunits of typhoid toxin (PltB, PltA, and CdtB) are secreted to the cis side of the periplasm by the sec pathway, where they assemble into the holotoxin complex (1). The muramidase TtsA introduces a fenestration in the YcbB-modified peptidoglycan layer at the bacterial poles allowing the passage of typhoid toxin to the trans side of the periplasmic space, positioning the toxin in close proximity to the outer membrane (2) from where it is released to the exterior upon minor disruptions to the outer membrane caused by various agonists encountered by S. Typhi during infection (3).