Table 1.
Classification of phenolic compounds in extra-virgin olive oil (EVOO).
| Phenolic Acids | ||
|---|---|---|
| Hydroxybenzoic Acid Derivatives |
p-Hydroxybenzoic acid (R1 = H; R2 = H) Protocatechuic acid (R1 = OH; R2 = H) Vanillic acid (R1 = OCH3; R2 = H) Syringic acid (R1 = OCH3; R2 = OCH3) Gallic acid (R1 = OH; R2 = OH) |
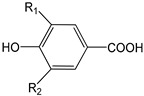
|
| Hydroxycinnamic Acid Derivatives |
p-Coumaric acid (R1 = H; R2 = H) Ferulic acid (R1 = OCH3; R2 = H) Caffeic acid (R1 = OH; R2 = H) Sinapic acid (R1 = OCH3; R2 = OCH3) |
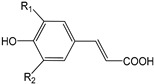
|
| Lignans | ||
| (+)-1-Acetoxypinoresinol (R1 = COOCH3; R2 = H; R3 = H) (+)-1-pinoresinol (R1 = H; R2 = H; R3 = H) |
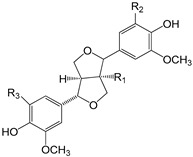
|
|
| Flavonoids | ||
| Luteolin (R1 = OH; R2 = H; R3 = H) Apigenin (R1 = H; R2 = H; R3 = H) |
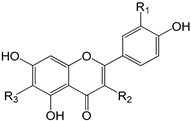
|
|
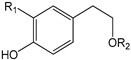
| Phenolic Alcohols | ||
| Hydroxytyrosol (R1 = OH; R2 = H) Tyrosol (R1 = H; R2 = H) |
|
|
| Secoiridoids | ||
| Oleuropein aglycone (R1 = OH; R2 = COOCH3; R3 = H) Ligstroside aglycone (R1 = H; R2 = COOCH3; R3 = H) Oleacein (R1 = OH; R2 = H; R3 = H) Oleocanthal (R1 = H; R2 = H; R3 = H) |
|
|
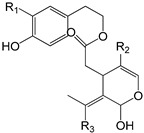
| Hydroxy-Isocromans | ||
| 1-phenyl-6,7-dihydroxy-isochroman 1-(39-methoxy-49-hydroxy) phenyl-6,7-dihydroxy-isochroman. |
||
