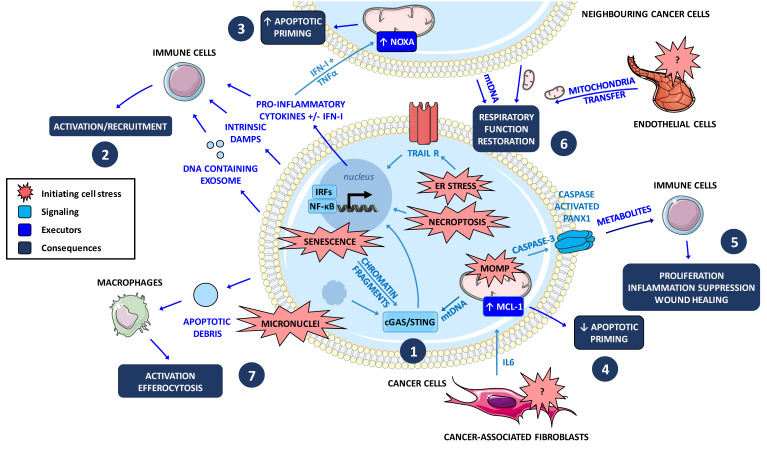
Figure 4.
Intrinsic cancer cell response and intratumoral dialogue induced by chemotherapies. 1. Senescence-dependent chromatin fragments, antimitotic-induced micronuclei or MOMP-released mtDNA contribute to proinflammatory secretory phenotypes in cancer cells treated by chemotherapies. Unprotected self DNA activate the cytosolic DNA sensor pathway cGAS-STING and downstream NF-kB and IFN-I signaling triggering chemo-induced secretome production. Chemo-induced necroptosis or ER stress (through intrinsic ligand-independent signaling) also promote a secretory phenotype. 2. Pro-inflammatory cytokines, interferons as well as intrinsic (constitutive or inducible) Damage Associated Molecular Patterns (DAMPs) or DNA-containing exosomes can activate and recruit immune cells. 3. Antimitotic-dependent secretome increases NOXA expression and apoptotic priming via TNFα/IFN-I in cancer cells in a paracrine manner. 4. Pro-inflammatory cytokines (IL-6) from stromal cells (CAF) reduce apoptotic priming in cancer cells through IL-6-dependent MCL-1 upregulation. 5. Active caspase-3 opens PANX1 channel releasing metabolites that potentially contribute to proliferation, inflammation suppression, and wound healing phenotype in neighboring healthy cells. 6. Mitochondria or mtDNA transfer from endothelial cell to cancer cell or between two cancer cells can repopulate mitochondrial network in committed cells and restore respiratory functions. 7. Apoptotic debris activate efferocytosis and pro-inflammatory cytokine secretion by macrophages.